The objective of this study was to determine if late use of aspirin before coronary artery bypass grafting (CABG) with valve surgery affects bleeding events and major adverse cardiovascular events. Aspirin has been shown to decrease postoperative CABG mortality and ischemic events. There are no data on the time of aspirin discontinuation and its effect on CABG with valve surgery and bleeding complications. From January 1, 2002 to January 31, 2008, 1,963 patients undergoing nonurgent plus valve surgery at the Cleveland Clinic were on preoperative aspirin; 1,404 (72%) discontinued aspirin ≥6 days before surgery (early discontinuation) and 559 (28%) continued aspirin within 5 days of surgery (late use). Propensity-score analysis and matching were employed for fair comparison of outcomes. There was no difference between early-discontinuation and late-use groups in the composite outcome of in-hospital mortality, myocardial infarction, and stroke (5.3% in the 2 groups). More patients in the late-use group received postoperative transfusions (49% vs 42%, p = 0.02). There was a trend toward increased reoperation for bleeding (6.1% vs 3.7%, p = 0.08) in the late-use group. In conclusion, in patients undergoing CABG with valve surgery, there was an increased use of postoperative red blood cell transfusion and a trend toward increased reoperation for bleeding in the late-use group. There was no difference in major adverse cardiac events between groups. Late use of aspirin in CABG with valve surgery must be weighed against an increased risk of bleeding.
Aspirin is a common therapy for patients with coronary artery disease. It has been shown to decrease postoperative coronary artery bypass grafting (CABG) mortality and myocardial ischemic events. However, there has been controversy on the timing of aspirin discontinuation before CABG owing to concerns for postoperative bleeding complications. Thus, we studied the effect of aspirin discontinuation earlier or later than 5 days before surgery on in-hospital mortality, myocardial infarction, stroke, and bleeding complications in patients undergoing CABG with valve surgery in the current era.
Methods
We analyzed data obtained from the Cardiovascular Information Registry (CVIR), a database of clinical and laboratory information on consecutive patients undergoing cardiothoracic surgery at the Cleveland Clinic. Data from this registry have been approved for use in research by the institutional review board with patient consent waived. All patients at the Cleveland Clinic sign a patient acknowledgment and consent form at admission, which allows the institution to use its clinical data for research to be published in aggregate and de-identified. This registry is certified for providing data to the Society of Thoracic Surgeons (STS) Adult Cardiac National Database. From January 1, 2002 to January 31, 2008, information on preoperative use of aspirin was prospectively collected in 1,963 patients undergoing nonurgent CABG with concomitant valve surgery at the Cleveland Clinic. Of these 1,404 (72%) discontinued aspirin ≥6 days before surgery (early discontinuation) and 559 (28%) used aspirin within 5 days of surgery (late use).
Our primary end point was a composite end point of in-hospital mortality, myocardial infarction, and stroke. Standard STS definitions of stroke and myocardial infarction were used. Myocardial infarction during hospitalization must fulfill 2 of the following 3 criteria: ischemic symptoms in the presence or absence of chest discomfort, enzyme increase, and/or ≥2 serial electrocardiograms showing ST-T waves changes from baseline. Ischemic symptoms can include chest, epigastric, arm, wrist, or jaw discomfort, unexplained nausea or vomiting, persistent dyspnea secondary to left ventricular failure and/or unexplained weakness, dizziness, lightheadedness, diaphoresis, or syncope. Enzyme increase requires at least creatine kinase-MB or creatine kinase >2 times the upper limit of normal, maximum creatine kinase-MB higher than the upper limit of normal on 2 successive samples, lactate dehydrogenase subtype 1 higher than subtype 2, or maximum troponin T or I higher than the limit for myocardial infarction at the institution during the first 24 hours after the event.
Our secondary end points were intraoperative red blood cell (RBC) transfusion, postoperative RBC transfusion, and return to the operating room for bleeding. Of note, there was no protocol followed to determine use of blood transfusion or when a patient underwent a reoperation. These decisions were left to the discretion of the surgeon. Our individual tertiary end points were postoperative myocardial infarction, postoperative stroke, and in-hospital mortality.
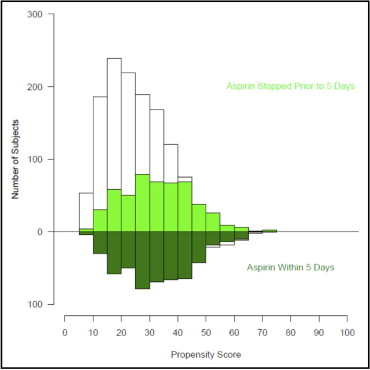
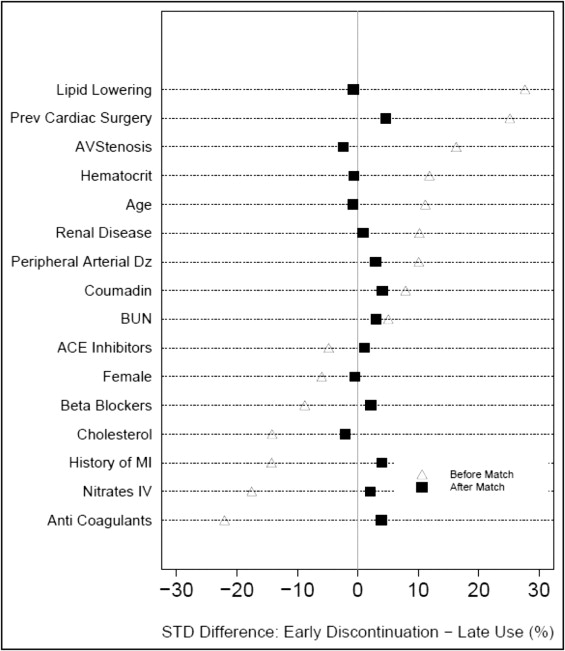
Because of substantial differences in characteristics between the 2 groups of patients, a propensity analysis was performed to decrease the bias when comparing outcomes. Multivariable logistic regression was used to identify patient factors associated with continued aspirin use within 5 days of surgery. Variables used for this analysis included patient demographics, symptoms, assessment of ventricular dysfunction, valve pathology, coronary anatomy, other cardiac co-morbidities, noncardiac co-morbidities, medications used, and surgical experience (time of operation since January 1, 1997). Variables used in our modeling were only those prospectively collected from the CVIR database. Bagging was used for variable selection based on the median rule (variables or closely clustered variables appearing with p <0.05 in ≥50% of 500 automated stepwise bootstrap models). Medication variables from the STS collection form were also added to the parsimonious model and propensity scores (probability of being in the late group) were calculated for each patient. Preoperative medications included were β blockers, angiotensin-converting enzyme inhibitors, intravenous nitrates, nonwarfarin anticoagulants (including unfractionated heparin, low-molecular-weight heparin, and direct thrombin inhibitors), warfarin, inotropes, steroids, and lipid-lowering agents. In all, 29 variables were included in the propensity model. Distribution of propensity score in each group is shown in Figure 1 . We adjusted for the probability of being in the late-use group. Using a greedy matching algorithm patients from each group were matched on similar propensity scores and 508 well-matched pairs were obtained. A standardized difference plot ( Figure 2 ) demonstrated that the groups were well matched for variables from the propensity model and additional demographic data.
Statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina). Descriptive summary data stratified by aspirin use (earlier than 5 days or within 5 days before CABG) are presented for all variables listed in Tables 1 and 2 . Continuous data are expressed as mean ± SD. Wilcoxon rank-sum tests were used to analyze differences for continuous data. Categorical data are displayed as frequency and percentage and comparisons were made using chi-square tests (or Fisher’s exact tests if appropriate).
| Early Discontinuation Aspirin Use >5 Days Before Surgery | Late Use Aspirin ≤5 Days Before Surgery | p Value | |||
|---|---|---|---|---|---|
| No. | Mean ± SD or No. (%) | No. | Mean ± SD or No. (%) | ||
| Age (years) | 1,404 | 71.3 ± 9.86 | 559 | 70.1 ± 10.6 | 0.08 |
| Women | 1,404 | 433 (31%) | 559 | 188 (34%) | 0.2 |
| Preoperative body mass index (kg/m 2 ) | 1,404 | 28.4 ± 5.51 | 559 | 28 ± 5.28 | 0.1 |
| Preoperative creatinine | 1,395 | 553 | 0.8 | ||
| mmol/L | 112 ± 96 | 110 ± 89.3 | |||
| mg/dl | 1.27 ± 1.09 | 1.24 ± 1.01 | |||
| Preoperative hematocrit (%) | 1,382 | 38.2 ± 5.73 | 555 | 37.5 ± 5.91 | 0.02 |
| Previous myocardial infarction | 1,404 | 689 (49%) | 559 | 314 (56%) | 0.005 |
| Previous stroke | 1,406 | 168 (12%) | 564 | 54 (9.7%) | 0.1 |
| Atrial fibrillation/flutter ⁎ | 1,404 | 265 (19%) | 559 | 81 (14%) | 0.02 |
| Previous infective endocarditis | 1,404 | 48 (3.4%) | 559 | 18 (3.2%) | 0.8 |
| Hypertension (history) | 1,403 | 1,174 (84%) | 558 | 459 (82%) | 0.4 |
| Treated diabetes mellitus | 1,403 | 420 (30%) | 559 | 163 (29%) | 0.7 |
| Carotid artery disease | 1,404 | 960 (68%) | 559 | 357 (64%) | 0.05 |
| Previous heart failure | 1,404 | 712 (51%) | 559 | 286 (51%) | 0.9 |
| Smoker (previous or current) | 1,403 | 849 (61%) | 559 | 334 (60%) | 0.8 |
| Renal disease † | 1,404 | 108 (7.7%) | 559 | 29 (5.2%) | 0.05 |
| Previous percutaneous coronary intervention | 1,404 | 390 (28%) | 559 | 128 (23%) | 0.03 |
| Previous percutaneous transluminal coronary angioplasty | 1,404 | 325 (23%) | 559 | 110 (20%) | 0.09 |
| Drug-eluting stent ‡ | 1,404 | 75 (5.3%) | 559 | 14 (2.5%) | 0.006 |
| Bare-metal stent ‡ | 1,404 | 73 (5.2%) | 559 | 23 (4.1%) | 0.30 |
| Stent (unknown type) | 1,404 | 177 (13%) | 559 | 72 (13%) | 0.90 |
| Previous cardiac surgery | 1,404 | 491 (35%) | 559 | 132 (24%) | <0.0001 |
| Nonwarfarin anticoagulants § | 1,386 | 368 (27%) | 555 | 204 (37%) | <0.0001 |
| Warfarin | 1,386 | 116 (8.4%) | 555 | 35 (6.3%) | 0.1 |
| Adenosine diphosphate inhibitor | 1,386 | 241 (17%) | 555 | 65 (12%) | 0.002 |
| β Blocker | 1,386 | 938 (68%) | 555 | 398 (72%) | 0.08 |
| Angiotensin-converting enzyme inhibitor | 1,386 | 877 (63%) | 555 | 364 (66%) | 0.3 |
| Intravenous nitrates | 1,386 | 120 (8.7%) | 555 | 79 (14%) | 0.0003 |
| Lipid-lowering medication | 1,386 | 619 (45%) | 555 | 174 (31%) | <0.0001 |
| Statin □ | 619 | 457 (74%) | 174 | 141 (81%) | 0.05 |
⁎ History of preoperative arrhythmia (atrial fibrillation, atrial flutter) that was clinically documented or treated with any of the following treatment methods: ablation therapy, an implantable cardioverter defibrillator, pacemaker, pharmacologic treatment, and/or electrocardioversion.
† History of renal disease defined as documented history of renal failure and/or history of creatinine level >2.0 mg/dl or on dialysis.
‡ Data on whether patients had drug-eluting or bare-metal stent began to be collected in 2004.
§ Nonwarfarin anticoagulants include unfractionated heparin, low-molecular-weight heparin, and direct thrombin inhibitors.
| Early Discontinuation Aspirin Use >5 Days Before Surgery | Late Use Aspirin ≤5 Days Before Surgery | p Value | |||
|---|---|---|---|---|---|
| No. | No. (%) | No. | No. (%) | ||
| Total number of previous cardiac operations | |||||
| 0 | 1,404 | 913 (65%) | 559 | 427 (76%) | <0.0001 |
| 1 | 396 (28%) | 111 (20%) | |||
| 2 | 86 (6.1%) | 20 (3.6%) | |||
| 3 or 4 | 9 (0.6%) | 1 (0.2%) | |||
| Number of coronary arteries with ≥50% stenosis | |||||
| 1 | 268 (20%) | 98 (18%) | 0.8 | ||
| 2 | 365 (27%) | 147 (27%) | |||
| 3 | 683 (50%) | 288 (52%) | |||
| Left main stenosis ≥50% | 1,359 | 298 (22%) | 543 | 117 (22%) | 0.9 |
| Number of internal thoracic grafts | |||||
| 0 | 1,403 | 634 (45%) | 559 | 207 (37%) | 0.001 |
| 1 | 752 (54%) | 338 (60%) | |||
| 2 | 17 (1.2%) | 14 (2.5%) | |||
| Multiple valve procedures | 1,404 | 256 (18%) | 559 | 95 (17%) | 0.5 |
| Previous valve operation | 1,404 | 103 (7.3%) | 559 | 33 (5.9%) | 0.3 |
| Aortic valve procedure | 1,404 | 932 (66%) | 559 | 338 (60%) | 0.01 |
| Aortic valve replacement | 1,404 | 916 (65%) | 559 | 325 (58%) | 0.003 |
| Aortic valve: mechanical prosthesis | 919 | 25 (2.7%) | 335 | 15 (4.5%) | 0.1 |
| Aortic valve: bioprosthesis | 919 | 869 (95%) | 335 | 301 (90%) | 0.003 |
| Aortic valve: homograft | 919 | 7 (0.8%) | 335 | 6 (1.8%) | 0.1 |
| Mitral valve procedure | 1,404 | 631 (45%) | 559 | 286 (51%) | 0.01 |
| Mitral valve repair | 1,404 | 451 (32%) | 559 | 213 (38%) | 0.01 |
| Mitral valve ring annuloplasty | 623 | 395 (63%) | 285 | 189 (66%) | 0.4 |
| Mitral valve replacement | 1,404 | 180 (13%) | 559 | 73 (13%) | 0.9 |
| Tricuspid valve procedure | 1,404 | 126 (9%) | 559 | 40 (7.2%) | 0.2 |
| Pulmonary valve procedure | 1,404 | 3 (0.2%) | 559 | 1 (0.2%) | 0.9 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


