Acute kidney injury (AKI) is a serious complication of cardiovascular surgery. Although some nonexperimental studies suggest that statin use may reduce postsurgical AKI, methodologic differences in study designs leave uncertainty regarding the reality or magnitude of the effect. The aim of this study was to estimate the effect of preoperative statin initiation on AKI after coronary artery bypass grafting (CABG) using an epidemiologic approach more closely simulating a randomized controlled trial in a large CABG patient population. Health care claims from large, employer-based and Medicare insurance databases for 2000 to 2010 were used. To minimize healthy user bias, patients were identified who underwent nonemergent CABG who either newly initiated a statin <20 days before surgery or were unexposed for ≥200 days before CABG. AKI was identified <15 days after CABG. Multivariate-adjusted risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using Poisson regression. Analyses were repeated using propensity score methods adjusted for clinical and health care utilization variables. A total of 17,077 CABG patients were identified. Post-CABG AKI developed in 3.4% of statin initiators and 6.2% of noninitiators. After adjustment, a protective effect of statin initiation on AKI was observed (RR 0.78, 95% CI 0.63 to 0.96). This effect differed by age, with an RR of 0.91 (95% CI 0.68 to 1.20) for patients aged ≥65 years and an RR of 0.62 (95% CI 0.45 to 0.86) for those aged <65 years, although AKI was more common in the older group (7.7% vs 4.0%). In conclusion, statin initiation immediately before CABG may modestly reduce the risk for postoperative AKI, particularly in younger CABG patients.
Statins may have anti-inflammatory and endothelial stabilizing pleiotropic effects with potential benefits on kidney function, motivating investigation into protective effects against postsurgical kidney injury. However, studies have used widely varying definitions of preoperative statin use, including prescribed statin use at the time of surgery, administration the day of or the day before surgery, and pharmacy dispensing <90 days before coronary artery bypass grafting (CABG). Many of these definitions fail to consider the history or duration of statin use, which may introduce bias due to the healthy user effect, whereby differences in healthy behaviors between long-term medication users and nonusers may lead to exaggerated estimates of the benefits of preventive medications. These important methodologic concerns limit the ability to distinguish whether observed results are a direct beneficial effect of statins or are caused by unmeasured behavioral differences in long-term users of statins. Yet acute kidney injury (AKI) remains a serious complication of CABG, resulting in short- and long-term consequences, including chronic kidney disease (CKD), end-stage renal disease, and death. Understanding the effect of preoperative statin use could allow clinicians to modify the risk for an outcome for which there are currently no proved interventions. In a cohort of patients who underwent planned CABG, we compared postsurgical AKI risk among those initiating statin therapy immediately before surgery with risk among those not initiating statins using a modern epidemiologic study design and analysis aimed at minimizing confounding bias.
Methods
Patients who underwent CABG surgery from 2000 to 2010 were identified in the MarketScan Commercial Claims and Encounters database and the MarketScan Medicare Supplemental and Coordination of Benefits database (Thomson Reuters, New York, New York). These databases are compilations of insurance billing data for employees, dependents, and retirees from across the United States with employer-based primary insurance (ages 18–64 years) or Medicare supplemental insurance coverage (aged ≥65 years). Adjudicated, paid inpatient, outpatient, and pharmacy claims, as well as enrollment information, are included in the databases. This study was exempted from further review by the University of North Carolina institutional review board.
All patients aged ≥18 years with inpatient procedure claims for CABG, with 200 days of continuous plan enrollment before hospital admission for CABG, were identified. If a patient had multiple eligible CABG procedures, only the first was considered. The 20 days immediately before the date of hospital admission were considered the exposure window, during which statin initiation was assessed. The 180 days before the exposure window were considered the washout period, during which the absence of any statin prescription was required (see Figure 1 ). We required ≥1 pharmacy claim for any nonstatin medication during the washout period to ensure pharmacy benefit utilization.
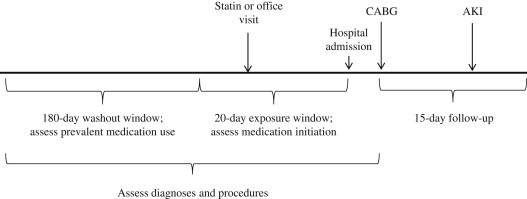
Patients with inpatient or outpatient diagnosis codes for AKI, unspecified renal failure, or end-stage renal disease in the 200 days before CABG were excluded. To restrict to planned CABG procedures by removing patients with emergency surgeries, we excluded patients with inpatient claims for myocardial infarction or unstable angina during the 20-day exposure period, CABG occurring after the fifth day of hospitalization, or angiographic studies in the 3 days before CABG.
Statin initiation was defined as having a pharmacy dispensing claim for any statin during the exposure window, without any statin claims during the preceding baseline period. Nonusers had no observable statin use during the exposure or baseline windows and were required to have an outpatient physician’s office visit during the exposure window to ensure health care utilization.
Baseline covariates for multivariate regression and propensity score (PS) models included claims for diagnoses, procedures, prevalent medication use, and preoperative initiation of other, nonstatin medications. Included covariates are listed in Table 1 . Baseline diagnoses and procedures were assessed in the 200 days before hospital admission for CABG and during the hospitalization up to the day of surgery and included age, gender, year of surgery, number of grafts in surgery, diagnoses of cardiovascular conditions, indicators of cardiovascular disease management, acute cardiovascular events and procedures, evidence of renal conditions, number of emergency department visits, and number of hospitalizations. Claims for medications during the washout period were considered prevalent medication use. If the medications were newly initiated during the exposure window without use during the washout period, the medications were considered newly initiated and considered separately in the analysis.
| Variable | Statin Noninitiators (n = 13,992) | Statin Initiators (n = 3,085) |
|---|---|---|
| Male | 10,394 (74.3%) | 2,453 (79.5%) |
| Age (yrs) | 65.4 ± 11.0 | 62.5 ± 10.0 |
| MarketScan database | ||
| Commercial Claims and Encounters | 7,135 (51.0%) | 1,978 (64.1%) |
| Medicare Supplemental and Coordination of Benefits | 6,857 (49.0%) | 1,107 (35.9%) |
| Health care utilization | ||
| Day of hospitalization on which CABG was performed | 0.88 ± 1.26 | 0.49 ± 0.99 |
| Number of lipid tests ∗ | 0.68 ± 1.11 | 0.84 ± 1.12 |
| Number of creatinine measurements ∗ | 0.04 ± 0.37 | 0.03 ± 0.25 |
| Number of hospitalizations ∗ | 0.67 ± 0.76 | 0.47 ± 0.70 |
| Number of emergency department visits ∗ | 0.14 ± 0.49 | 0.11 ± 0.43 |
| Cardiovascular disease management | ||
| Angiography performed | 10,492 (75.0%) | 2,634 (85.4%) |
| Cardiac stress test performed | 8,381 (59.9%) | 2,166 (70.2%) |
| Echocardiography performed | 8,030 (57.4%) | 1,723 (55.9%) |
| Cardiovascular and co-morbid conditions | ||
| Number of vessels bypassed during surgery | ||
| 1 or 2 | 5,265 (37.6%) | 951 (30.8%) |
| 3–5 | 7,565 (54.1%) | 1,779 (57.7%) |
| ≥6 | 862 (6.2%) | 259 (8.4%) |
| Diabetes mellitus | 4,170 (29.8%) | 813 (26.4%) |
| CKD | 109 (0.8%) | 13 (0.4%) |
| Other kidney disease | ||
| Proteinuria | 70 (0.5%) | 13 (0.4%) |
| Hypertension | 7,208 (51.5%) | 1,596 (51.7%) |
| Hyperlipidemia | 4,550 (32.5%) | 1,283 (41.6%) |
| Other ischemic heart disease | 12,811 (91.6%) | 2,972 (96.3%) |
| Atrial fibrillation | 1,422 (10.2%) | 163 (5.3%) |
| Acute events in previous 6 months | ||
| Recent myocardial infarction † | 484 (3.5%) | 108 (3.5%) |
| History of myocardial infarction | 364 (2.6%) | 83 (2.7%) |
| Unstable angina † | 1,743 (12.5%) | 464 (15.0%) |
| Stroke | 3,723 (26.6%) | 746 (24.2%) |
| Insertion of a coronary stent | 236 (1.7%) | 51 (1.7%) |
| Angioplasty | 228 (1.6%) | 50 (1.6%) |
| Prevalent medication use during baseline | ||
| Angiotensin-converting enzyme inhibitors | 4,349 (31.1%) | 916 (29.7%) |
| Angiotensin receptor blockers | 2,212 (15.8%) | 434 (14.1%) |
| β blockers | 4,981 (35.6%) | 982 (31.8%) |
| Calcium channel blockers | 3,219 (23.0%) | 607 (19.7%) |
| Antiplatelet agents | 1,497 (10.7%) | 231 (7.5%) |
| α blockers | 1,324 (9.5%) | 200 (6.5%) |
| Thiazide diuretics | 3,215 (23.0%) | 675 (21.9%) |
| Potassium-sparing diuretics | 880 (6.3%) | 124 (4.0%) |
| Loop diuretics | 1,794 (12.8%) | 210 (6.8%) |
| Niacin | 250 (1.8%) | 36 (1.2%) |
| Fibrates | 976 (7.0%) | 150 (4.9%) |
| Ezetimibe | 637 (4.6%) | 85 (2.8%) |
| Anticoagulants | 1,031 (7.4%) | 106 (3.4%) |
| Nonsteroidal anti-inflammatory drugs | 2,612 (18.7%) | 562 (18.2%) |
| Medications initiated during exposure window | ||
| Angiotensin-converting enzyme inhibitors | 1,410 (10.1%) | 606 (19.6%) |
| Angiotensin receptor blockers | 573 (4.1%) | 189 (6.1%) |
| β blockers | 2,832 (20.2%) | 1,498 (48.6%) |
| Calcium channel blockers | 928 (6.6%) | 306 (9.9%) |
| Antiplatelet agents | 549 (3.9%) | 253 (8.2%) |
| α blockers | 546 (3.9%) | 248 (8.0%) |
| Thiazide diuretics | 839 (6.0%) | 246 (8.0%) |
| Potassium-sparing diuretics | 249 (1.8%) | 52 (1.7%) |
| Loop diuretics | 634 (4.5%) | 106 (3.4%) |
| Niacin | 69 (0.5%) | 49 (1.6%) |
| Fibrates | 264 (1.9%) | 57 (1.9%) |
| Ezetimibe | 143 (1.0%) | 176 (5.7%) |
| Anticoagulants | 308 (2.2%) | 47 (1.5%) |
| Nonsteroidal anti-inflammatory drugs | 456 (3.3%) | 78 (2.5%) |
∗ Occurring <200 days before admission for CABG.
† Not including events that occurred in the 20 days before hospital admission for CABG, because those patients were excluded.
Inpatient claims in the 15 days after CABG were searched for International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for AKI (584.5 to 584.9). Sensitivity analyses were performed using a broader definition of kidney failure (any of the following diagnosis codes: acute renal failure [584.5 to 584.9], end-stage renal disease [585.6], or unspecified renal failure [586]).
We estimated the association between statin initiation and AKI using multivariate Poisson regression, resulting in adjusted risk ratios (RRs) and 95% confidence intervals (CIs). We also performed regression analyses using stabilized inverse probability of treatment weighting. Multivariate logistic regression was used to estimate the predicted probability of initiating a statin, or PS, for each patient in the sample using the prespecified covariates described previously. To exclude patients treated contrary to prediction, as their extreme weights may disproportionately influence the effect measure estimate, we trimmed patients with PS less than the 1st percentile of the treated or greater than the 99th percentile of the untreated. The PS was then used to calculate the inverse probability of treatment weighting in the remaining patients, and the weights were applied to a Poisson regression model.
Last, we performed 1-to-1 PS matching using a greedy matching algorithm whereby nonusers were matched to statin initiators by PS to the fifth decimal place, if possible. We then estimated RRs using regression models in the remaining matched patients.
Models were run separately in the 2 databases: commercial insurance (ages 40 to 64 years) and Medicare supplementary insurance (ages ≥65 years). We also repeated the analyses in prespecified subgroups: by gender and in those without CKD. Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
Sensitivity analyses were performed by varying the length of the exposure window before CABG (10 to 30 days) to observe if the effect may be dependent on the length of time on statin before CABG. To estimate the extent to which medication initiation is simply a proxy for better presurgical care, the entire analysis was repeated considering β-blocker initiation as a negative control exposure rather than statin initiation. Beta blockers are preventive cardiovascular medications with a similar behavioral profile and user population as statins, but they are not thought to confer a protective effect against postoperative AKI. Therefore, if a protective effect was observed among the β-blocker initiators, it could be assumed that our study design did not adequately address the healthy user effect and other sources of confounding bias.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


