We aimed to describe the impact of the vascular access used when patients are treated with primary percutaneous coronary intervention (PPCI) and to assess whether this translates into differences in angiographic outcomes. Patients with ST-elevation myocardial infarction who underwent PPCI were divided into 3 groups: successful radial access (RA), successful femoral access (FA), and Crossover (failed RA with need for bailout FA) groups. Vascular access–related time (VART) was defined as the delay in PPCI that can be attributed to vascular access–related issues. Study end point was the final corrected Thrombolysis In Myocardial Infarction frame count. Multivariable analysis was used to identify predictors of RA failure (RAF: FA + Crossover). We included 241 patients (RA, n = 172; FA, n = 49; Crossover, n = 20). Mean VART was longer in Crossover (10.3 [8.8 to 12.4] minutes), relative to RA (4.1 [3.2 to 5.5] minutes) and FA (4.6 [3.4 to 8.4] minutes, p <0.001). A similar situation was found for time-to-first device (Crossover 22.5 [20.3 to 32.0], RA 15.0 [12.0 to 19.8]; FA 17.9 [13.5 to 22.3] minutes, p <0.001) and total procedure time (Crossover 60.3 [51.6 to 71.5], RA 46.8 [38.1 to 59.7], FA 52.3 [41.9 to 74.7] minutes, p <0.001). No differences in corrected Thrombolysis In Myocardial Infarction frame count were observed (Crossover 26 [18 to 32] frames, RA 24 [18 to 32] frames, FA 25 [16 to 34] frames, p = 0.625). Killip class IV (odds ratio [OR] 3.628, 95% confidence interval [CI] 1.098 to 11.981, p = 0.035), cardiopulmonary resuscitation before arrival (OR 3.572, 95% CI 1.028 to 12.407, p = 0.045), and glomerular filtration rate (OR 0.861, 95% CI 0.758 to 0.978, p = 0.021) were independent predictors of RA failure. In conclusion, in the setting of PPCI, radial-to-FA crossover can lead to VART delays that do not affect angiographic outcomes, in comparison with successful RA.
Highlights
- •
Radial access (RA) can be attempted in 80% of patients with ST-elevation myocardial infarction.
- •
RA is successfully secured in 90% of the cases when it is attempted.
- •
RA failure is associated with small delays that do not affect outcomes.
- •
Killip class IV, resuscitation before arrival, and impaired renal function predict RA failure.
Radial access (RA) has recently gained popularity among interventionalists as it is associated with reduced bleeding and access-site complications, compared with femoral access (FA). In particular, patients with ST-elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PPCI) through RA in the RadIal Vs. femorAL access for coronary intervention (RIVAL) trial experienced a 40% reduction in the primary end point (death, myocardial infarction, stroke, and major bleeding). Similar findings have been observed also in other trials (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome (RIFLE-STEACS) and ST Elevation Myocardial Infarction treated by RADIAL or femoral approach (STEMI-RADIAL) ) and in large registries. However, current practice in North America still favors FA as the default access route for PPCI because it is believed to be quicker to obtain and eases catheter manipulation, thus reducing total procedure time and radiation dose. In STEMI, prompt restoration of coronary flow is critical. Concerns over securing RA and navigating the tortuous arm and subclavian vasculature are part of a steep learning curve, which could explain the slow uptake of transradial PPCI in many centers. Therefore, we aimed to describe the characteristics of patients with STEMI treated with PPCI according to vascular access and to compare if there are significant differences among study groups. Finally, we also sought to identify predictors of RA failure (RAF).
Methods
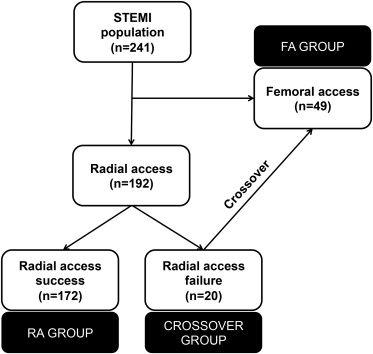
The study population included patients with STEMI treated with PPCI (rescue and facilitated PCI cases were excluded). A total of 241 consecutive patients admitted at our institution from April 2012 to March 2013 were included. Figure 1 depicts study workflow. Clinical, biochemical, angiographic, and echocardiographic variables and also data on PPCI and adverse events were collected. This study was approved by the institutional review board. Patients were divided into 3 groups and analyzed accordingly: (1) procedure was intended a priori and successfully performed through the FA (FA group), (2) procedure was intended a priori and successfully performed through the RA (RA group), and (3) procedure was intended a priori and attempted through the RA, but failed, with subsequent crossover to FA (Crossover group).
The outcome of this study was the final corrected Thrombolysis In Myocardial Infarction (TIMI) frame count (CTFC), as assessed off-line by a trained operator. The CTFC is the number of cine frames required for dye to first reach standardized distal coronary landmarks and quantifies the efficacy of reperfusion. As previously reported by Gibson et al, lower CTFCs in the setting of STEMI are associated with improved outcomes after reperfusion.
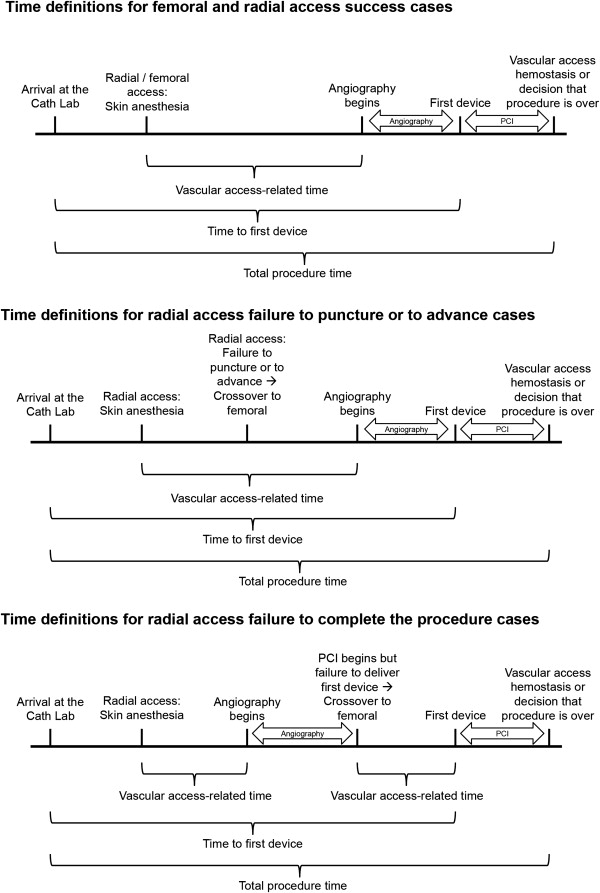
Three reasons for radial-to-femoral crossover were identified: (1) inability to puncture the radial artery; (2) RA is obtained, but the guidewire/catheter cannot advance (e.g., due to severe vasospasm or tortuosity); (3) diagnostic catheterization is successfully performed, but PCI is not possible (e.g., because of lack of support: inability to complete the procedure). Vascular access–related time (VART) was defined as the delay in PPCI that is attributed to access-related issues. Figure 2 describes how VART and other key time variables were calculated for successful RA or FA procedure and for each Crossover scenario.
Major adverse cardiac events (MACEs) were assessed during hospital stay and were defined as a composite of all-cause death, reinfarction, and target vessel revascularization. In-hospital major adverse events were defined as a composite of MACEs, stroke, heart failure, Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) major bleeding, and Acute Kidney Injury Network (AKIN) acute kidney injury.
Analyses were carried out to ascertain whether significant differences existed among groups. Normality of variable distribution was tested with the Shapiro-Wilk test. In case of normality, continuous variables are presented as mean ± SD and 1-way analysis of variance was used. Otherwise, continuous variables presented as median (first to third quartile) and nonparametric tests, such as the Kruskal-Wallis test, were used for comparisons. Categorical variables are presented as frequency (%) and compared using either the chi-square or Fisher’s exact test, when appropriate. We also sought to identify predictors of RAF, which was defined as either decision to directly choose an FA (FA group) or need for bailout FA after failed RA access (Crossover group). A stepwise logistic regression analysis was used to identify independent predictors of RAF, and all variables showing a p ≤0.15 on univariate analysis were included. For all tests, a p <0.05 was considered significant. Statistical analysis was performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina).
Results
As shown in Figure 1 , RA was attempted in 80% of our STEMI population (n = 192). RA was secured in 172 patients (RA group). Crossover was needed in the remaining 20 cases (10% of RA attempts). Table 1 lists the baseline characteristics of the population. Mean age was 62.2 ± 12.6 years. The study population was predominantly composed by men, with 16% being patients with diabetes. About a third of STEMI location was anterior. Patients of the Crossover and FA group tended to be older than the subjects of the RA group (p = 0.055) and were more likely to have hypertension (p = 0.069) and previous heart failure (p = 0.079). Similarly, patients of the FA group had worse renal function (p <0.001) and presented more frequently with signs of heart failure (p <0.001) and cardiac arrest (p <0.001). Overall clinical severity was higher in patients of the FA group as reflected by different risk scores.
| Variable | Overall (n = 241) | RA (n = 172) | FA (n = 49) | Crossover (n = 20) | p for Interaction | |
|---|---|---|---|---|---|---|
| Age (years) | 62.2 ± 12.6 | 61.0 ± 12.3 | 65.9 ± 12.0 | 63.5 ± 16.1 | 0.055 | |
| Men | 186 (77%) | 137 (80%) | 36 (73%) | 13 (65%) | 0.264 | |
| Body mass index (kg/m 2 ) | 28.1 ± 5.1 | 28.4 ± 5.1 | 26.8 ± 4.3 | 28.5 ± 6.3 | 0.185 | |
| Hypertension | 121 (50%) | 79 (46%) | 28 (57%) | 14 (70%) | 0.069 | |
| Hyperlipidemia | 127 (53%) | 84 (49%) | 31 (63%) | 12 (60%) | 0.161 | |
| Diabetes mellitus | 39 (16%) | 23 (13%) | 12 (24%) | 4 (20%) | 0.157 | |
| Current smoking | 101 (42%) | 76 (44%) | 16 (33%) | 9 (45%) | 0.338 | |
| Family history of CAD | 70 (29%) | 52 (30%) | 11 (22%) | 7 (35%) | 0.473 | |
| Prior PCI | 26 (11%) | 15 (9%) | 9 (18%) | 2 (10%) | 0.157 | |
| Prior CABG | 4 (2%) | 0 | 4 (8%) | 0 | <0.001 | |
| Prior heart failure | 8 (3%) | 3 (2%) | 4 (8%) | 1 (5%) | 0.079 | |
| Prior stroke | 13 (5%) | 8 (5%) | 4 (8%) | 1 (5%) | 0.629 | |
| Prior chronic kidney disease | 18 (7%) | 10 (6%) | 7 (14%) | 1 (5%) | 0.128 | |
| Glomerular filtration rate (ml/min/1.73 m 2 ) | 74.0 ± 28.7 | 78.3 ± 27.4 | 56.0 ± 23.4 | 80.1 ± 34.5 | <0.001 | |
| Systolic blood pressure (mm Hg) | 118 ± 26 | 120 ± 26 | 111 ± 28 | 122 ± 22 | 0.097 | |
| STEMI location | Anterior | 88 (37%) | 61 (35%) | 20 (41%) | 7 (35%) | 0.264 |
| Inferior | 136 (56%) | 101 (59%) | 24 (49%) | 11 (55%) | ||
| Lateral | 7 (3%) | 4 (2%) | 1 (2%) | 2 (10%) | ||
| Other (posterior, LBBB, pacemaker) | 10 (4%) | 6 (3%) | 4 (8%) | 0 | ||
| Killip class | I | 205 (85%) | 161 (94%) | 26 (53%) | 18 (90%) | <0.001 |
| II | 7 (3%) | 3 (2%) | 2 (4%) | 2 (10%) | ||
| III | 6 (2%) | 2 (1%) | 4 (8%) | 0 | ||
| IV | 23 (10%) | 6 (4%) | 17 (35%) | 0 | ||
| Cardiac arrest before arrival | 20 (8%) | 7 (4%) | 12 (24%) | 1 (5%) | <0.001 | |
| GRACE risk score | 165 ± 46 | 154 ± 36 | 208 ± 56 | 160 ± 37 | <0.001 | |
| TIMI risk score | 3 (1–5) | 3 (1–5) | 5 (3–7) | 2.5 (1–5.5) | <0.001 | |
Table 2 lists the procedural characteristics of the population. No differences in culprit artery and the number of diseased vessel were observed. However, coronary artery disease (CAD) burden, as quantified by the SYNTAX score, was higher in patients of the FA group (p = 0.002). This was also hinted by a lower prevalence of aspirin loading, because patients were already on that medication for known CAD (p <0.001). Patients of the FA group presented more frequently with a TIMI flow grade 0 to 1 (p = 0.008). Periprocedural medications and PPCI techniques were similar among groups. Finally, as our institution is a teaching hospital with a thriving interventional cardiology fellowship program, we explored whether fellow experience affected procedural times. There was no significant association between crossover rates and the level of fellow experience (p = 0.371) or specific fellow (p = 0.303). The Supplementary Table 1 analyzes the relation between operator experience and procedural metrics: although the former was associated with better utilization of cath laboratory resources (contrast volume, radiation dose, fluoroscopy time), no differences were found for the primary end point (final CTFC).
| Variable | Overall (n = 241) | RA (n = 172) | FA (n = 49) | Crossover (n = 20) | p for Interaction | |
|---|---|---|---|---|---|---|
| Arrival at the cath lab between midnight and 8 am | 51 (21%) | 30 (17%) | 15 (31%) | 6 (30%) | 0.087 | |
| Culprit vessel | Right | 117 (49%) | 83 (48%) | 25 (51%) | 9 (45%) | 0.203 |
| LAD | 93 (39%) | 63 (37%) | 21 (43%) | 9 (45%) | ||
| Cx | 28 (12%) | 25 (15%) | 1 (2%) | 2 (10%) | ||
| LM | 2 (0.8%) | 1 (0.6%) | 1 (1%) | 0 | ||
| >1 artery | 1 (0.4%) | 0 | 1 (2.0%) | 0 | ||
| Vessels diseased | 1 | 134 (56%) | 101 (59%) | 22 (45%) | 11 (55%) | 0.125 |
| 2 | 69 (29%) | 48 (28%) | 16 (33%) | 5 (25%) | ||
| 3 | 23 (10%) | 17 (10%) | 3 (6%) | 3 (15%) | ||
| 1 + LM | 3 (1%) | 2 (1%) | 1 (2%) | 0 | ||
| 2 + LM | 5 (2%) | 2 (1%) | 3 (6%) | 0 | ||
| 3 + LM | 7 (3%) | 2 (1%) | 4 (8%) | 1 (5%) | ||
| LM alone | 0 | 0 | 0 | 0 | ||
| SYNTAX score | 12.0 (8.0–19.0) | 11.0 (7.0–17.5) | 19.0 (10.0–24.0) | 10.0 (8.5–17.3) | 0.002 | |
| Aspirin loading dose | 232 (96%) | 170 (99%) | 42 (86%) | 20 (100%) | <0.001 | |
| P2Y 12 loading dose | 237 (98%) | 169 (98%) | 48 (98%) | 20 (100%) | 0.823 | |
| Anticoagulant | Heparin | 225 (94%) | 164 (95%) | 44 (93%) | 17 (85%) | 0.156 |
| Bivalirudin | 15 (6%) | 8 (5%) | 4 (8%) | 3 (15%) | ||
| Periprocedural glycoprotein IIb/IIIa inhibitor | 78 (32%) | 56 (33%) | 14 (29%) | 8 (40%) | 0.651 | |
| Initial TIMI flow grade | 0 | 160 (67%) | 111 (65%) | 38 (79%) | 11 (55%) | 0.056 |
| 1 | 13 (5%) | 7 (4%) | 5 (10%) | 1 (5%) | ||
| 2 | 27 (11%) | 22 (13%) | 1 (2%) | 4 (20%) | ||
| 3 | 40 (17%) | 32 (19%) | 4 (8%) | 4 (20%) | ||
| Initial TIMI flow grade 0–1 | 173 (72%) | 118 (69%) | 43 (90%) | 12 (60%) | 0.008 | |
| Thrombectomy | 195 (82%) | 142 (83%) | 39 (85%) | 14 (70%) | 0.329 | |
| Direct stenting | 109 (48%) | 83 (50%) | 17 (40%) | 9 (53%) | 0.421 | |
| Postdilatation | 115 (52%) | 85 (52%) | 21 (50%) | 9 (56%) | 0.913 | |
| Type of PCI | POBA | 21 (9%) | 14 (8%) | 3 (6%) | 4 (21%) | 0.142 |
| BMS | 133 (57%) | 90 (53%) | 33 (72%) | 10 (53%) | ||
| DES | 77 (33%) | 62 (37%) | 10 (22%) | 5 (26%) | ||
| BVS | 3 (1%) | 3 (2%) | 0 | 0 | ||
| Fellow experience | ≤400 cases | 83 (34%) | 56 (33%) | 21 (43%) | 6 (30%) | 0.371 |
| >400 cases | 158 (66%) | 116 (67%) | 28 (57%) | 14 (70%) | ||
| Vascular access-related time (minutes) | 4.4 (3.3–6.5) | 4.1 (3.2–5.5) | 4.6 (3.4–8.4) | 10.3 (8.8–12.4) | <0.001 | |
| Time to first device (minutes) | 16.0 (13.0–21.0) | 15.0 (12.0–19.8) | 17.9 (13.5–22.3) | 22.5 (20.3–32.0) | <0.001 | |
| Total procedure time (minutes) | 50.3 (38.8–62.9) | 46.8 (38.1–59.7) | 52.3 (41.9–74.7) | 60.3 (51.6–71.5) | <0.001 | |
| Contrast volume (ml) | 200 (170–250) | 200 (170–250) | 200 (160–230) | 240 (190–265) | 0.124 | |
| Radiation dose (cGy·cm 2 ) | 9,238 (6,318–14,959) | 8,727 (6,287–14,262) | 9,422 (6,463–13,136) | 17,283 (6,770–19,852) | 0.029 | |
| Fluoroscopy time (minutes) | 10.4 (7.2–16.2) | 9.8 (6.6–15.2) | 10.2 (7.3–16.4) | 15.7 (10.1–19.3) | 0.058 | |
| Final TIMI flow grade | 0 | 3 (1%) | 2 (1%) | 1 (2%) | 0 | 0.637 |
| 1 | 1 (0.4%) | 1 (0.6%) | 0 | 0 | ||
| 2 | 32 (13%) | 19 (11%) | 10 (21%) | 3 (15%) | ||
| 3 | 202 (85%) | 149 (87%) | 36 (77%) | 17 (85%) | ||
| Final CTFC | 24 (18–32) | 24 (18–32) | 25 (16–34) | 26 (18–32) | 0.625 | |
| No reflow | 24 (10%) | 16 (9%) | 5 (11%) | 3 (15%) | 0.719 | |
| Peak high-sensitivity troponin T (ng/l) | 3,151 (1,345–7,485) | 3,061 (853–7,367) | 3,644 (2,245–9,379) | 2,625 (1,824–5,658) | 0.262 | |
| Peak CK-MB (U/l) | 77.5 (8.8–186.7) | 81.5 (8.8–181.8) | 63.8 (5.7–312.0) | 88.0 (40.7–186.7) | 0.916 | |
| Ejection fraction (%) | 47.8 ± 9.6 | 48.6 ± 8.7 | 43.9 ± 11.8 | 51.5 ± 6.7 | 0.023 | |
As shown in Figure 3 , time outcomes were longer in patients of the Crossover group, compared with the other groups. More specifically, VART was 10.3 (8.8 to 12.4) minutes in Crossover, 4.6 (3.4 to 8.4) minutes in FA, and 4.1 (3.2 to 5.5) minutes in RA (p <0.001). Similarly, differences were found for patients of the Crossover group with regard to radiation dose (p = 0.029) and fluoroscopy time (p = 0.058), as shown in Figure 4 . Conversely, no difference was observed for the final CTFC (Crossover: 26 [18 to 32] frames, FA: 25 [16 to 34] frames, RA: 24 [18 to 32] frames, p = 0.625). Figure 5 shows the distribution of the final CTFC in the 3 groups. There were also no differences for other angiographic (final TIMI flow grade and presence of no reflow) and biochemical (peak troponin and CK-MB) outcomes.
In a post hoc analysis, given an alpha error of 5%, RA (n = 172), RAF (FA + Crossover, n = 69), a common final CTFC SD of 14.1 frames, an important difference in final CTFC ≥10, and a 2-tailed hypothesis, the power of our study was 99.9%.
Table 3 lists in-hospital adverse events. The incidence of adverse events was high in the FA group as reflected by high-risk scores in this patient population ( Table 1 ). In particular, MACEs occurred more often in the FA (25%) than in the RA (4%, p <0.001) and Crossover (5%, p = 0.060) groups: this was driven by a higher incidence of both death and reinfarction. In the FA group, major adverse events were also more frequent (43%) than in the RA (10%, p <0.001) and Crossover (20%, p = 0.073) groups. Specifically, this was mainly because of greater occurrences of acute heart failure, acute kidney injury, and major bleeding. The only significant difference between the RA and Crossover groups was related to the occurrence of major bleeding, which was higher in patients of the Crossover group (10% vs 0.6%, p = 0.002).



