The influence of preinfarction angina pectoris (AP) on long-term clinical outcomes in patients with ST-segment elevation myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention (PCI) remains controversial. In 5,429 patients with acute myocardial infarction (AMI) enrolled in the Coronary Revascularization Demonstrating Outcome Study in Kyoto AMI Registry, the present study population consisted of 3,476 patients with STEMI who underwent primary PCI within 24 hours of symptom onset and in whom the data on preinfarction AP were available. Preinfarction AP defined as AP occurring within 48 hours of hospital arrival was present in 675 patients (19.4%). Patients with preinfarction AP was younger and more often had anterior AMI and longer total ischemic time, whereas they less often had history of heart failure, atrial fibrillation, and shock presentation. The infarct size estimated by peak creatinine phosphokinase was significantly smaller in patients with than in patients without preinfarction AP (median [interquartile range] 2,141 [965 to 3,867] IU/L vs 2,462 [1,257 to 4,495] IU/L, p <0.001). The cumulative 5-year incidence of death was significantly lower in patients with preinfarction AP (12.4% vs 20.7%, p <0.001) with median follow-up interval of 1,845 days. After adjusting for confounders, preinfarction AP was independently associated with a lower risk for death (hazard ratio 0.69, 95% confidence interval 0.54 to 0.86, p = 0.001). The lower risk for 5-year mortality in patients with preinfarction AP was consistently observed across subgroups stratified by total ischemic time, initial Thrombolysis In Myocardial Infarction flow grade, hemodynamic status, infarct location, and diabetes mellitus. In conclusion, preinfarction AP was independently associated with lower 5-year mortality in patients with STEMI who underwent primary PCI.
Highlights
- •
The prevalence of preinfarction AP was 19.4% in patients with STEMI who underwent PCI.
- •
Preinfarction AP was associated with limited infarct size.
- •
Preinfarction AP was independently associated with a lower 5-year mortality.
- •
Mortality in patients with preinfarction AP was less affected by total ischemic time.
Preinfarction angina pectoris (AP) before the onset of ST-segment elevation myocardial infarction (STEMI) was reported to be associated with limited infarct size and improved clinical outcomes in patients with STEMI in the thrombolytic era in accordance with the previous experimental studies reporting the protective effect of brief episodes of ischemia before coronary occlusion on infarct size. The prevalence of preinfarction AP in previous studies, however, varies widely from 11% to 69% according to the definitions of preinfarction AP. Furthermore, the cardioprotective effects of preinfarction AP in patients with STEMI are still controversial in the primary percutaneous coronary intervention (PCI) era. The clinical significance of preinfarction AP was evaluated in several relatively small studies, and most of those studies evaluated infarct size instead of mortality with discordant results in those studies. Therefore, we sought to assess the clinical significance of preinfarction AP on long-term mortality of patients with STEMI who underwent primary PCI in a large, multicenter acute myocardial infarction (AMI) registry in Japan.
Methods
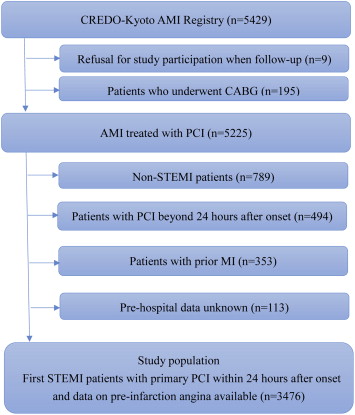
The Coronary Revascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) AMI registry is a physician-initiated, non–company-sponsored multicenter registry that enrolled consecutive patients with AMI having coronary revascularization within 7 days of the onset of symptoms from January 2005 to December 2007 across 26 tertiary hospitals in Japan ( Supplementary Appendix A ). The relevant review boards or ethics committees in all 26 participating centers approved the study protocol. Obtaining written informed consent from the patients was waived because of the retrospective study design. In 5,429 patients enrolled in the CREDO-Kyoto AMI registry, 9 patients were excluded because of their refusal to participate in the study when contacted at follow-up. We also excluded 195 patients who had coronary artery bypass grafting surgery, 789 patients with non-STEMI, 494 patients who received PCI beyond 24 hours after onset, 353 patients with previous myocardial infarction (MI), and 113 patients in whom data on the presence or absence of preinfarction AP were not available because their clinical histories before the onset of STEMI were not found in their charts or database ( Figure 1 ). We excluded patients with previous MI because previous studies have reported that cardioprotective mechanism of ischemic preconditioning is impaired in patients with postinfarct left ventricular remodeling. Therefore, the study population for the current analysis consisted of 3,476 patients with first STEMI who had primary PCI within 24 hours of onset and in whom data on the presence or absence of preinfarction AP were available.
Experienced clinical research coordinators in the independent research organization (Research Institute for Production Development, Kyoto, Japan) collected demographic, angiographic, and procedural data from the hospital charts or hospital databases according to the prespecified definitions ( Supplementary Appendix B ). They also collected data about symptoms within 48 hours before hospital arrival from the hospital charts or hospital databases.
Collection of follow-up information was mainly conducted through review of the inpatient and outpatient hospital charts by the clinical research coordinators, and additional follow-up information was collected through contact with patients, relatives, and/or referring physicians by sending mail with questions regarding vital status, subsequent hospitalizations, and status of antiplatelet therapy. Serious adverse events, such as death, MI, stent thrombosis, and stroke, were adjudicated by the clinical event committee ( Supplementary Appendix C ). The median length of follow-up in this study was 1,845 (interquartile range [IQR]: 1,517 to 2,155) days.
Preinfarction AP was defined as typical AP, chest discomfort, or radiating pain persisting <30 minutes within 48 hours of hospital arrival for the index STEMI. Atypical chest pain or dyspnea was not included in preinfarction AP because it was difficult to assess whether those symptoms were representing myocardial ischemia. Those patients whose symptoms of preinfarction AP were not described in their charts or database despite available clinical information before hospital arrival were regarded as those without preinfarction AP. The detailed definitions of other baseline clinical characteristics were described previously.
The primary outcome measure for this analysis was all-cause death. Death was regarded as cardiac in origin unless obvious noncardiac causes could be identified. Any death during the index hospitalization for AMI was regarded as cardiac death. As the secondary outcome measure, recurrent MI was also evaluated. MI was defined according to the definition in the Arterial Revascularization Therapy Study.
We present continuous variables as the mean and SD or median and IQR and categorical variables as numbers and percentages. We compared continuous variables with the Student’s t test or a Wilcoxon rank-sum test on the basis of the distributions. We compared categorical variables with the chi-square test when appropriate; otherwise, we used the Fisher’s exact test. A multivariable logistic regression model was used to identify independent predictors of preinfarction AP.
We used the Kaplan-Meier method to estimate the cumulative incidences of clinical event and assessed the differences with the log-rank test. The effects of preinfarction AP for the primary outcome measure were expressed as hazard ratios with 95% confidence intervals by multivariable Cox proportional hazard models adjusting for the 36 clinically relevant factors indicated in Table 1 . We did not use hemodynamic status, infarct size, and total ischemic time as covariates in the multivariable models because these factors could potentially be affected by the presence of preinfarction AP. We also computed the adjusted cumulative incidence curves of patients with and without preinfarction AP using a multivariable Cox proportional hazards model in conjunction with the methods described by Ghali et al. Consistent with our previous reports, continuous variables were dichotomized using clinically meaningful reference values or median values. We also evaluated the effects of preinfarction AP on the primary outcome measure in several subgroups stratified by total ischemic time (≤3 hours, 3 to 6 hours, or 6 to 24 hours), Thrombolysis In Myocardial Infarction (TIMI) flow grade (0 or ≥1), hemodynamic status (Killip class I or Killip class II to IV), infarct location (anterior MI or nonanterior MI), and diabetes mellitus (diabetes mellitus or non-diabetes mellitus). For the subgroup analyses, we also developed the Cox proportional hazard models incorporating the same risk adjusting variables to estimate the effect of preinfarction AP for the primary outcome measure.
| Variable | Pre-infarction Angina Pectoris | p Value | |
|---|---|---|---|
| Yes (N = 675) | No (N = 2801) | ||
| Age (years ± SD) | 66.1 ± 11.7 | 67.5 ± 12.4 | 0.008 |
| Age ≥ 75 years ∗ | 174 (26%) | 866 (31%) | 0.009 |
| Male ∗ | 492 (73%) | 2036 (73%) | 0.92 |
| Body mass index † (mean ± SD) | 23.5 ± 3.4 | 23.7 ± 3.5 | 0.26 |
| Body mass index <25 kg/m 2 ∗ | 485 (72%) | 2010 (72%) | 0.96 |
| Hypertension ∗ | 539 (80%) | 2163 (77%) | 0.14 |
| Diabetes mellitus | 196 (29%) | 878 (31%) | 0.24 |
| On insulin therapy ∗ | 24 (3.6%) | 120 (4.3%) | 0.39 |
| Current smoker ∗ | 290 (43%) | 1145 (41%) | 0.32 |
| Prior heart failure ∗ | 4 (0.6%) | 53 (1.9%) | 0.02 |
| Severe mitral regurgitation ∗ | 8 (1.2%) | 79 (2.8%) | 0.01 |
| Prior stroke (symptomatic) ∗ | 53 (7.9%) | 242 (8.6%) | 0.51 |
| Peripheral vascular disease ∗ | 17 (2.5%) | 85 (3.0%) | 0.48 |
| eGFR ‡ <30 ml/min/1.73 m 2 , without dialysis ∗ | 18 (2.7%) | 122 (4.4%) | 0.045 |
| Hemodialysis ∗ | 9 (1.3%) | 39 (1.4%) | 0.91 |
| Atrial fibrillation ∗ | 40 (5.9%) | 277 (9.9%) | 0.001 |
| Anemia (Hb < 11.0 g/dl) ∗ | 60 (8.9%) | 260 (9.3%) | 0.75 |
| Thrombocytopenia (PLT <10*10 4 ) ∗ | 9 (1.3%) | 54 (1.9%) | 0.30 |
| COPD ∗ | 30 (4.4%) | 83 (3.0%) | 0.051 |
| Liver cirrhosis ∗ | 17 (2.5%) | 65 (2.3%) | 0.76 |
| Malignancy ∗ | 48 (7.1%) | 220 (7.9%) | 0.52 |
| Hours from onset to presentation | 3.1 (1.3–7.4) | 2.3 (1.1–4.9) | <0.001 |
| Hours from onset to balloon | 4.9 (3.1–9.1) | 4.1 (2.8–6.9) | <0.001 |
| ≤3 hours from onset to balloon | 145/595 (24%) | 728/2460 (30%) | 0.01 |
| Minutes from door to balloon | 90 (60–132) | 90 (60–132) | 0.66 |
| Hemodynamics | |||
| Killip class 1 | 567 (84%) | 2061 (74%) | <0.001 |
| Killip class 2 | 48 (7.1%) | 227 (8.1%) | |
| Killip class 3 | 7 (1.0%) | 79 (2.8%) | <0.001 |
| Killip class 4 (cardiogenic shock) | 53 (7.9%) | 434 (15%) | <0.001 |
| Killip class 2–4 | 108 (16%) | 740 (26%) | |
| Location of MI | |||
| Anterior wall | 363 (54%) | 1320 (47%) | <0.001 |
| Inferior wall | 224 (33%) | 1161 (41%) | |
| Lateral wall | 16 (2.4%) | 86 (3.1%) | <0.001 |
| Posterior wall | 72 (11%) | 234 (8.4%) | 0.006 |
| TIMI flow grade 0 | 367 (54%) | 1687 (60%) | |
| Multivessel disease ∗ | 322 (48%) | 1387 (50%) | 0.40 |
| Target of proximal LAD ∗ | 419 (62%) | 1513 (54%) | <0.001 |
| Target of unprotected LMCA ∗ | 24 (3.6%) | 97 (3.5%) | 0.91 |
| Target of CTO ∗ | 14 (2.1%) | 91 (3.3%) | 0.11 |
| Target of bifurcation ∗ | 197 (29%) | 723 (26%) | 0.07 |
| Side-branch stenting ∗ | 22 (3.3%) | 87 (3.1%) | 0.84 |
| Total stent length >28 mm ∗ | 272 (42%) | 1082 (42%) | 0.97 |
| Minimum stent size <3.0 mm ∗ | 220 (34%) | 799 (31%) | 0.16 |
| Medications at discharge | |||
| Thienopyridine | 657 (97%) | 2663 (95%) | 0.01 |
| Aspirin | 670 (99%) | 2749 (98%) | 0.04 |
| Cilostazole ∗ | 254 (38%) | 969 (35%) | 0.14 |
| Statin ∗ | 374 (55%) | 1487 (53%) | 0.28 |
| Beta-blockers ∗ | 282 (42%) | 1181 (42%) | 0.86 |
| ACE-I/ARB ∗ | 515 (76%) | 2011 (72%) | 0.02 |
| Nitrates ∗ | 255 (38%) | 780 (28%) | <0.001 |
| Calcium channel blockers ∗ | 125 (19%) | 550 (20%) | 0.51 |
| Nicorandil ∗ | 196 (29%) | 777 (28%) | 0.50 |
| Warfarin ∗ | 62 (9.2%) | 309 (11%) | 0.16 |
| Proton pump inhibitors ∗ | 242 (36%) | 955 (34%) | 0.39 |
| H2-blockers ∗ | 235 (35%) | 910 (32%) | 0.25 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


