The present study sought to assess the effectiveness of local anesthesia with conscious sedation (LACS) during transcatheter aortic valve implantation (TAVI). On its introduction, TAVI was mostly performed with the patient under general anesthesia (GA); however, evidence supporting the use of less-invasive LACS has been increasing. The data from 174 consecutive patients who underwent TAVI by way of the femoral artery from December 2007 to December 2011 were analyzed. GA was mainly used in early phase of the study (n = 44); this was gradually shifted to LACS in the late phase (n = 130). The clinical outcomes were compared for those patients who received GA versus LACS. The incidence and causes of “LACS failure,” defined as conversion to GA from LACS during TAVI, were also assessed. The rates of procedural success and 30-day mortality were not different between the 2 groups (93.3% vs 95.3%, p = 0.60; 6.7% vs 7.8%, p = 0.55, respectively). Although the clinical backgrounds of the patients showed differences, these results were not significant after adjusting for other influential confounders. The intensive care unit stay and hospital stay were longer in the GA group than in the LACS group (3.9 ± 2.2 vs 3.3 ± 1.5 days, p = 0.044; and 12.2 ± 8.3 vs 8.1 ± 6.5 days, p = 0.001, respectively). LACS failure occurred in 6 patients (4.6%), and the causes were multifactorial, as follows: cardiac tamponade in 2, cardiac arrest in 2, myocardial infarction in 1, and stroke in 1. In conclusion, transfemoral TAVI with the patient under LACS could be successfully performed in most patients, with the advantage of early recovery, although the perioperative risks involved in the TAVI procedure should be considered.
Transcatheter aortic valve implantation (TAVI) has emerged as a novel alternative procedure that enables catheter-based treatment in high-risk surgical aortic valve replacement patients. The technique of TAVI is thought to be reaching maturity, although several clinical problems associated with the treatment of such high-risk patients remain, with the anesthetic management during TAVI being controversial. Soon after the introduction of TAVI, general anesthesia (GA) with endotracheal intubation was usually chosen, despite differences in the approach site used for this technique. Recently, a few investigations have demonstrated the feasibility of TAVI with the patient under local anesthesia with conscious sedation (LACS), owing to the increased experience of the technique and device improvements. However, the data for TAVI under LACS and the incidence of conversion to GA from LACS during TAVI, defined as “LACS failure,” are limited. Therefore, the aim of the present study was to assess the differences in clinical outcomes between GA and LACS during TAVI and to elucidate the frequency and causes of LACS failure.
Methods
From December 2007 to December 2011, 182 consecutive patients who were diagnosed with symptomatic severe aortic stenosis and assessed to be at high surgical risk underwent TAVI at the Henri Mondor University Hospital. Of these patients, 8 underwent surgery using nontransfemoral access sites (subclavian artery in 5 and carotid artery in 3) and were excluded from the initial analysis. The data from the remaining 174 patients were prospectively investigated for differences in the anesthetic method used. The definition of “severe” aortic stenosis was determined by the echocardiographic findings of an aortic valve area of <0.8 cm 2 or 0.6 cm 2 /m 2 , a peak aortic jet velocity of >4.0 m/s, or a mean aortic valve gradient of >40 mm Hg. All patients were screened before TAVI to determine whether they were considered unsuitable for surgical aortic valve replacement, according to a consensus between cardiac surgeons and cardiologists. Information on survival was obtained from the treating hospital or by a telephone call made directly to the patient or the patient’s family. All clinical data, patient characteristics, transthoracic echocardiographic results, transesophageal echocardiographic (TEE) results, procedural parameters, and intensive care unit and hospital stay lengths were examined from the patients’ medical records, including their anesthetic charts. The medical ethics committee at Henri Mondor University Hospital, Val-de-Marne University, approved the study protocol, and all patients provided written informed consent before the TAVI procedure.
Detailed technical aspects of the TAVI procedure have been previously reported. Two TAVI systems are commercially available: a self-expandable prosthesis, the Medtronic CoreValve Revalving System (Medtronic, Minneapolis, Minnesota) and a balloon-expandable prosthesis, the Edwards Sapien valve (Edwards Lifesciences, Irvine, California). After insertion of a large 18F or 19F sheath into the femoral artery, unfractionated heparin was injected to maintain the activated coagulation time at >250 seconds. Rapid right ventricular pacing (range 160 to 200/min) was performed during balloon dilatation of the aortic valve. The femoral artery was closed using a percutaneous suture device (Proster XL, Abbott, Chicago, Illinois), except in cases of difficulties or failure of the suture device, in which case, the closure was performed surgically. Device success and 30-day combined safety data were evaluated according to the Valve Academic Research Consortium criteria. Other procedural complications during TAVI were also assessed according to the Valve Academic Research Consortium classification. The combined safety end point was defined as follows: all-cause mortality, major stroke, life-threatening bleeding, acute kidney injury stage 3, periprocedural myocardial infarction, major vascular complications, and a repeat procedure for valve-related dysfunction (surgical or interventional therapy).
All anesthetic preprocedural evaluations and procedural management were performed by well-experienced cardiac anesthesiologists. Extensive monitoring consisted of a 6-electrode, virtual 12-lead electrocardiogram, pulse oximetry, and invasive artery pressure measurement from the sheath. GA was introduced with a bolus of anesthetic (propofol, 2.5 μg/ml) and opioid derivative (remifentanil, 1.5 ng/ml), and a muscle relaxant (atracurium, 0.3 to 0.6 mg/kg), if required. All GA patients were intubated orally using a single lumen tube and treated under mechanical ventilation. Next, the TEE probe was inserted. For LACS, 1% lidocaine was applied subcutaneously at the femoral vascular access site (maximum limit 400 mg). GA was maintained under deep sedation with both a target-controlled infusion to reach the effect site concentration of propofol (1.5 μg/ml, increasing or decreasing) and a target-controlled infusion of remifentanil (0.5 ng/ml, increasing or decreasing). LACS was also maintained by a target-controlled infusion of remifentanil and/or a target-controlled infusion of propofol adjusted according to the response (Ramsay score 2 to 3).
All statistical analyses were performed using SPSS software, version 19 (SPSS, Chicago, Illinois). Continuous variables are expressed as the mean ± SD or median, depending on the variable distribution. Categorical data are expressed as a percentage of the total. Comparison between the 2 groups was performed using the chi-square test or the unpaired Student t test, as appropriate. Univariate regression analysis was performed to obtain the odds ratios for procedural success, 30-day mortality and 30-day combined safety end point after TAVI. Next, multivariate regression analysis was performed that included age, gender, and different baseline characteristics (i.e., dyslipidemia, peripheral artery disease, ejection fraction, and logistic European System for Cardiac Operative Risk Evaluation [euroSCORE]) to examine the independent association between anesthetic method and clinical outcomes. All statistical tests were 2-sided, and p <0.05 was considered significant.
Results
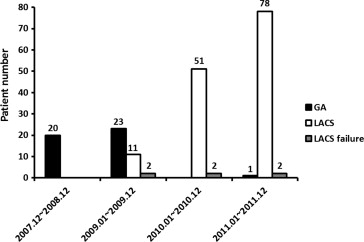
During the early phase of the physician learning curve for TAVI from December 2007 to December 2009, GA was mainly used because the operator considered the requirement for transesophageal echocardiography during the procedure important. After this phase, from January 2010 to December 2011, most patients underwent TAVI under LACS. In 2011, 1 patient started TAVI under GA because of peripheral artery disease and the possibility of a switch to a nontransfemoral approach during the procedure. A total of 44 patients underwent TAVI under GA, and 130 patients underwent TAVI with LACS. LACS failure occurred in 6 patients ( Figure 1 ). The baseline patient characteristics are listed in Table 1 . Patients with peripheral artery disease and dyslipidemia were more prevalent in the LACS group than in the GA group (p = 0.035 and p = 0.045, respectively). The ejection fraction of the left ventricle was significantly lower in the GA group than in the LACS group (45.1 ± 12.6% vs 50.4 ± 14.1%, p = 0.030). Thus, both the logistic EuroSCORE and the Society of Thoracic Surgeons predictive risk of mortality score were significantly greater in the GA group than in the LACS group (p = 0.029 and p = 0.002, respectively). The procedural characteristics are listed in Table 2 . The Medtronic CoreValve Revalving System (n = 160) was mainly chosen at our center, although the Edwards Sapien valve was implanted in 13 patients. The prevalence of procedural complications was similar between the 2 groups. The total procedure time, length of hospital stay, and length of intensive care unit stay were longer in the GA group than in the LACS group (p = 0.002, p = 0.001, and p = 0.044, respectively). No significant differences were seen in procedural success, 30-day mortality, or the 30-day combined safety end point (p = 0.60, p = 0.55, and p = 0.99, respectively). The baseline comorbidities showed some differences between the 2 groups; however, these results were not attenuated, even after adjusting for considerable influential factors (i.e., age, gender, dyslipidemia, peripheral artery disease, ejection fraction, and logistic EuroSCORE). On multivariate analysis, TAVI under LACS was not associated with an increasing risk of procedural success (odds ratio 0.91, 95% confidence interval 0.16 to 5.04, 0.97, p = 0.91), 30-day mortality (odds ratio 1.54, 95% confidence interval 0.36 to 6.57, p = 0.56), or the 30-day combined safety end point (odds ratio 1.02, 95% confidence interval 0.34 to 2.93, p = 0.96).
| Overall (n = 174) | GA (n = 44) | LACS (n = 130) | p Value | |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age (yrs) | 83.9 ± 7.1 | 84.7 ± 7.0 | 83.7 ± 7.1 | 0.43 |
| Men | 72 (41.4%) | 21 (46.7%) | 51 (39.5%) | 0.40 |
| Height (cm) | 162.7 ± 8.2 | 162.9 ± 8.5 | 162.6 ± 8.2 | 0.87 |
| Weight (kg) | 68.2 ± 13.7 | 69.4 ± 14.7 | 67.8 ± 13.3 | 0.51 |
| Body mass index (kg/m 2 ) | 25.7 ± 4.8 | 26.0 ± 4.0 | 25.7 ± 5.0 | 0.70 |
| Body surface area (m 2 ) | 1.7 ± 0.18 | 1.7 ± 0.21 | 1.7 ± 0.17 | 0.53 |
| New York Heart Association class (III/IV) | 114 (65.5%) | 28 (62.2%) | 86 (66.7%) | 0.59 |
| Peripheral artery disease | 32 (18.4%) | 13 (28.9%) | 19 (14.7%) | 0.035 |
| Previous heart failure | 112 (64.4%) | 29 (64.4%) | 83 (64.3%) | 0.99 |
| Previous myocardial infarction | 29 (16.7%) | 8 (17.8%) | 21 (16.3%) | 0.82 |
| Previous cerebrovascular event | 18 (10.3%) | 5 (11.1%) | 13 (10.1%) | 0.85 |
| Previous cardiac surgery | 27 (15.5%) | 7 (15.6%) | 20 (15.5%) | 0.99 |
| Diabetes mellitus | 39 (22.4%) | 6 (13.3%) | 33 (25.6%) | 0.065 |
| Hypertension | 138 (79.3%) | 33 (73.3%) | 105 (81.4%) | 0.25 |
| Dyslipidemia | 92 (52.9%) | 18 (40.0%) | 74 (57.4%) | 0.045 |
| Former smoker | 38 (21.8%) | 14 (31.8%) | 24 (18.5%) | 0.064 |
| Chronic obstructive pulmonary disease | 41 (23.6%) | 11 (24.4%) | 30 (23.3%) | 0.87 |
| Chronic kidney disease | 117 (67.2%) | 28 (63.6%) | 89 (68.5%) | 0.56 |
| Left ventricle ejection fraction <40% | 55 (31.6%) | 19 (42.2%) | 36 (27.9%) | 0.057 |
| Logistic European System for Cardiac Operative Risk Evaluation | 23.8 ± 11.9% | 26.6 ± 13.0% | 22.0 ± 11.9% | 0.031 |
| Society of Thoracic Surgeons Predictive Risk of Mortality score | 11.5 ± 8.1% | 14.3 ± 9.4% | 11.2 ± 7.8% | 0.033 |
| Echocardiographic data | ||||
| Left ventricular ejection fraction | 49.1 ± 13.9% | 45.1 ± 12.6% | 50.4 ± 14.1% | 0.030 |
| Aortic valve area (cm 2 ) | 0.68 ± 0.19 | 0.72 ± 0.16 | 0.67 ± 0.20 | 0.098 |
| Mean gradient (mm Hg) | 47.2 ± 17.0 | 41.6 ± 15.8 | 49.1 ± 17.0 | 0.010 |
| Aortic regurgitation grade ≥2 | 24 (14.0%) | 3 (6.8%) | 21 (16.2%) | 0.077 |
| Mitral regurgitation grade ≥2 | 23 (13.4%) | 8 (18.2%) | 15 (11.5%) | 0.22 |
| Pulmonary hypertension | 97 (56.4%) | 25 (56.8%) | 72 (56.3%) | 0.95 |
| Post aortic valve area (cm 2 ) | 2.0 ± 0.46 | 2.1 ± 0.47 | 1.9 ± 0.46 | 0.12 |
| Post mean gradient (mm Hg) | 8.8 ± 3.6 | 8.6 ± 3.3 | 8.9 ± 3.7 | 0.76 |
| Post aortic regurgitation grade ≥2 | 24 (14.0%) | 3 (6.8%) | 21 (16.2%) | 0.077 |
| Post mitral regurgitation grade ≥2 | 23 (13.4%) | 8 (18.2%) | 15 (11.5%) | 0.22 |
| Overall (n = 174) | GA (n = 44) | LACS (n = 130) | p Value | |
|---|---|---|---|---|
| Periprocedural variables | ||||
| Total procedure time (min) | 82.0 ± 30.6 | 93.5 ± 26.9 | 78.2 ± 30.9 | 0.002 |
| Fluoroscopy time (min) | 19.2 ± 9.5 | 16.2 ± 6.1 | 19.4 ± 10.7 | 0.063 |
| Contrast medium volume (ml) | 183.7 ± 96.5 | 180.7 ± 88.0 | 184.7 ± 99.6 | 0.81 |
| Valve type | 0.023 | |||
| Medotronic CoreValve | 160 (92.0%) | 44 (100.0%) | 116 (89.2%) | |
| Edwards Sapien | 14 (8.0%) | 0 (0.0%) | 14 (10.8%) | |
| Postprocedural variables | ||||
| Length of hospital stay (days) | 9.1 ± 7.1 | 12.2 ± 8.3 | 8.1 ± 6.5 | 0.001 |
| Length of intensive care unit stay (days) | 3.5 ± 1.7 | 3.9 ± 2.2 | 3.3 ± 1.5 | 0.044 |
| Procedural success | 165 (94.8%) | 42 (93.3%) | 123 (95.3%) | 0.60 |
| 30-Day mortality | 13 (7.5%) | 3 (6.7%) | 10 (7.8%) | 0.55 |
| 30-Day combined safety end point | 27 (15.5%) | 6 (15.6%) | 21 (15.5%) | 0.99 |
| Myocardial infarction | 2 (1.1%) | 1 (2.3%) | 1 (0.8%) | 0.44 |
| Major and minor stroke | 12 (6.9%) | 4 (8.9%) | 8 (6.2%) | 0.38 |
| Major stroke | 3 (1.7%) | 1 (2.3%) | 2 (1.5%) | 0.59 |
| Acute kidney injury grade ≥2 | 28 (16.3%) | 9 (20.0%) | 19 (15.0%) | 0.43 |
| Major vascular complication | 13 (7.5%) | 3 (6.7%) | 10 (7.8%) | 0.55 |
| Red blood cell transfusion | 29 (16.7%) | 10 (22.2%) | 19 (14.7%) | 0.25 |
| 2-Valve implantation | 5 (2.9%) | 2 (4.4%) | 3 (2.3%) | 0.39 |
| Pacemaker implantation | 31 (17.8%) | 10 (22.2%) | 21 (16.3%) | 0.37 |
| Cardiac tamponade | 2 (1.1%) | 0 (0.0%) | 2 (1.5%) | 0.56 |
| Ventricular fibrillation | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | >0.99 |
| Cardiogenic shock | 6 (2.3%) | 1 (2.3%) | 5 (3.8%) | 0.62 |
| Adrenergic agent requirement | 13 (7.5%) | 4 (9.1%) | 9 (6.9%) | 0.43 |
| Surgery for vascular complication | 8 (4.6%) | 3 (6.7%) | 5 (3.9%) | 0.34 |
| Any cardiac surgery | 4 (2.3%) | 1 (2.2%) | 3 (2.3%) | 0.97 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


