The aim of the present study was to investigate whether the implementation of an early invasive strategy for unselected patients with acute myocardial infarction (AMI) would be associated with reduced long-term mortality compared to a conservative approach. In this prospective observational cohort study of consecutive patients admitted for AMI in 2003 (conservative cohort, n = 311) and 2006 (invasive cohort [IC], n = 307), an 11% absolute and 41% relative reduction in 1-year mortality was found for patients with AMI in the IC compared to the conservative cohort (p = 0.001). These findings were consistent after adjustment for age, gender, previous AMI, previous stroke, diabetes, smoking status, previous left ventricular systolic dysfunction, and serum creatinine at admission (hazard ratio 0.54, 95% confidence interval 0.38 to 0.78) and Global Registry of Acute Coronary Events risk score (hazard ratio 0.67, 95% confidence interval 0.46 to 0.97). More patients with ST-segment elevation myocardial infarction received primary percutaneous coronary intervention in the IC (57% vs 3%, p <0.001), and a sixfold (25% vs 4%, p <0.001) increase in early percutaneous coronary intervention (<72 hours) for patients with non–ST-segment elevation myocardial infarction was observed. A greater proportion of patients in the IC received clopidogrel, aspirin, and statins during follow-up; otherwise, the secondary prevention measures were similar in the 2 cohorts. In conclusion, the introduction of a strategy for routine transfer to a high-volume percutaneous coronary intervention center for early invasive therapy was accompanied by a substantial reduction in mortality among unselected patients with AMI. Differences in unmeasured confounders might have accounted for a part of the difference in outcome.
In a study of unselected patients with acute myocardial infarction (AMI) treated in 2003 with a conservative regimen in our department, the 1-year mortality rates were greater than those reported in clinical trials and registries. In September 2005, we implemented new guidelines, including transportation of patients with ST-segment elevation myocardial infarction (STEMI) to a high-volume invasive center located 100 km (63 miles) from our hospital for primary percutaneous coronary intervention. Patients with non–STEMI (NSTEMI) underwent invasive procedures within 48 to 72 hours, regardless of evidence of ongoing myocardial ischemia. The aim of the present study was to investigate whether the introduction of an early invasive management strategy for unselected patients with AMI was associated with reduced long-term mortality compared to a conservative strategy.
Methods
During 2 1-year periods, all patients referred to our hospital with a suspected AMI were prospectively registered. The diagnosis of AMI was made in accordance with the European Society of Cardiology/American College of Cardiology criteria from 2000. The conservative strategy cohort (CC) included patients admitted from February 1, 2003 through January 31, 2004. The invasive strategy cohort (IC) included patients admitted from February 15, 2006 through February 14, 2007. In 2003, the catchment population totaled 126,000 inhabitants. In September 2006, it was increased to 165,000 but from an area of similar risk and socioeconomic status. The baseline characteristics included an estimation of the Global Registry of Acute Coronary Events risk score for 6-month mortality in both cohorts. All patients were offered participation in a 6-week cardiac rehabilitation program.
A diagnosis of AMI was made in the presence of typical symptoms and elevated troponin T to greater than a cutoff level of ≥0.1 μg/L in both cohorts. The subtype of AMI was classified according to the electrocardiographic findings. STEMI was considered present if persistent ST-segment elevation occurred in 2 adjacent leads (>0.1 mV in limb leads, >0.2 mV in V 1 to V 3 , and >0.1 mV in V 4 to V 6 ). Patients qualifying for AMI but presenting without persistent ST-segment elevation were classified as having NSTEMI. In the presence of left bundle branch block, patients were categorized as having STEMI if left bundle branch block was presumed to be of recent onset and otherwise as having NSTEMI.
A 12-lead electrocardiogram was recorded by paramedics for all patients with a suspected AMI and sent by telemetry to our coronary care unit for analysis by the physician on duty. Prehospital activation of the invasive center was done by this physician.
Reperfusion therapy for patients with STEMI in the CC was determined by fibrinolysis, preferably administered in the ambulance. Patients in the IC with a symptom duration of <12 hours and a transfer time to the invasive center <90 minutes were scheduled for primary percutaneous coronary intervention. Otherwise, eligible patients were treated with fibrinolysis. In both cohorts, patients treated with fibrinolytic therapy with <50% ST-segment recovery and/or recurrent symptoms after 60 minutes were transferred for rescue-percutaneous coronary intervention. Patients with successful fibrinolysis in the CC underwent ischemia-driven diagnostics and were referred for coronary angiography in the presence of symptoms and/or objective evidence of ischemia. Patients in the IC routinely underwent coronary angiography within 24 to 48 hours after fibrinolysis.
For patients with NSTEMI in the CC a “cool-down” policy was applied according to the guidelines. Only those with ongoing ischemic symptoms accompanied by ST-segment depression and/or negative T waves were transferred for invasive management within the first 48 to 72 hours. Patients in the CC underwent a submaximal exercise electrocardiogram at discharge and a maximal test after 6 weeks and were referred for coronary angiography in the case of positive findings and/or ischemic symptoms. The patients in the IC were referred for coronary angiography within 48 to 72 hours, regardless of symptoms or evidence of ongoing ischemia, provided dementia or severe co-morbidities were absent.
The diagnostic and therapeutic procedures were performed using standard techniques, mainly through radial access. In patients with STEMI, only the culprit lesion was treated in the acute setting, unless cardiogenic shock was present. Other lesions were treated after some weeks if clinically indicated. For patients with NSTEMI, all lesions were (in principle) treated with stenting whenever technically possible. In patients with advanced age or severe co-morbidities, complete revascularization procedures were not always performed. Patients with extensive triple vessel disease, including left main or proximal left anterior descending artery stenosis, were referred for surgery, which was performed within 1 to 7 days if not contraindicated.
In the CC, 147 patients (47%) and in the IC, 154 patients (50%) refused or were not considered capable of follow-up visits owing to dementia and/or severe co-morbidity. We confirmed the vital status for all patients included in the present study, regardless of their follow-up status. Because of regulatory restrictions, the causes of death were not available. Data were collected from the invasive center for all patients, including patients bypassing our hospital en route for primary percutaneous coronary intervention.
The regional ethics committee for South-East Norway Regional Health Authority and the Norwegian Social Science Data Services approved the study.
The Mann-Whitney U test was used for the comparison of continuous data between different groups of patients. Proportions were analyzed using the chi-square test or Fisher’s exact test. Two-tailed p values <0.05 were considered statistically significant. Kaplan-Meier plots and log-rank tests were used for comparison of survival between different subsets of patients. Cox proportional hazards regression models were used for additional survival analyses. In these analyses, the cohort was used as a surrogate variable for the treatment strategy. The assumption of proportional hazards was explored with partial residual plots. Interaction terms between cohort/age, cohort/smoking, and previous AMI/previous left ventricular systolic dysfunction were included and tested. An a priori power analysis was performed before the inclusion of patients in the second cohort (IC). The study had a power >80% (α = 0.05) to demonstrate a reduced mortality of 40% for NSTEMI and 35% for NSTEMI and STEMI combined. The analyses were implemented using the Statistical Package for Social Sciences, version 16.0 (SPSS, Chicago, Illinois).
Results
In 2003 (the CC period), a total of 755 patients were admitted to our hospital with a clinical suspicion of AMI, of whom 126 (17%) had STEMI and 185 (25%) had NSTEMI. The corresponding numbers in 2006 (the IC period) were 934, 107 (11%), and 200 (21%). Accordingly, the incidence of AMI in our catchment area was 247/100,000 inhabitants in 2003 and 220/100,000 inhabitants in 2006. The baseline characteristics of the patients with AMI in both cohorts are presented in Table 1 . A nonsignificant tendency was seen for older patients with STEMI in the CC, but no such difference was observed for NSTEMI or for all patients with AMI (median age 74 years, interquartile range 23, vs 72 years, interquartile range 24, p = 0.61). Creatinine was slightly greater in the CC, for both STEMI and NSTEMI. Otherwise, the baseline characteristics were comparable in the 2 cohorts, including the Global Registry of Acute Coronary Events risk score, which could be obtained for 94% of participants in both cohorts (median 133 in the CC vs 125 in the IC, p = 0.054).
| Variable | STEMI | NSTEMI | ||||
|---|---|---|---|---|---|---|
| CC (n = 126) | IC (n = 107) | p Value | CC (n = 185) | IC (n = 200) | p Value | |
| Age (years) | 69 (25) | 62 (23) | 0.13 | 76 (20) | 76 (23) | 0.91 |
| Male gender | 81 (64%) | 75 (70%) | 0.42 | 115 (62%) | 121 (61%) | 0.82 |
| Current smoker ⁎ | 47% | 45% | 0.87 | 30% | 25% | 0.29 |
| Diabetes mellitus | 12 (10%) | 14 (13%) | 0.52 | 27 (15%) | 35 (18%) | 0.53 |
| Previous acute myocardial infarction | 14 (11%) | 18 (17%) | 0.28 | 57 (31%) | 65 (33%) | 0.81 |
| Previous left ventricular systolic dysfunction † | 3 (2%) | 2 (2%) | 1.00 | 17 (9%) | 22 (11%) | 0.70 |
| Hypertension | 34 (27%) | 21 (20%) | 0.22 | 64 (35%) | 58 (29%) | 0.29 |
| Stroke | 4 (3%) | 6 (6%) | 0.56 | 12 (7%) | 19 (10%) | 0.40 |
| Coronary bypass | 5 (4%) | 1 (1%) | 0.30 | 17 (9%) | 23 (12%) | 0.57 |
| Coronary angioplasty | 4 (3%) | 6 (6%) | 0.56 | 9 (5%) | 20 (10%) | 0.086 |
| S-creatinine (μmol/L ‡ ) | 87 (28) | 78 (37) | 0.021 | 95 (51) | 87 (34) | 0.028 |
⁎ Smoking within previous 3 months.
† Defined as previous left ventricular ejection fraction <40%.
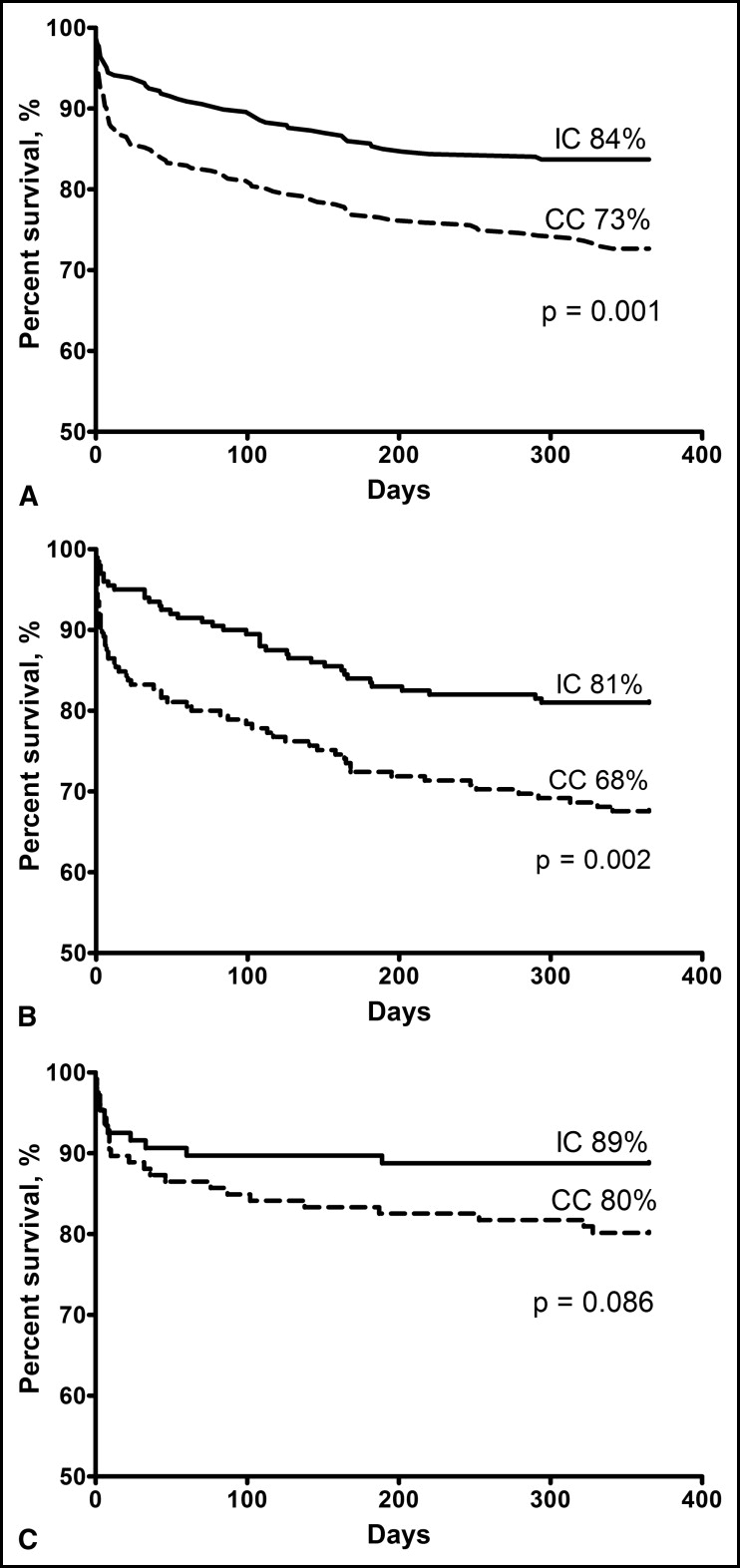
The Kaplan-Meier estimates of patient survival are shown in Figure 1 . For all patients with AMI, the 1-year mortality rate was 16% in the IC and 27% in the CC, with a 41% relative and 11% absolute risk reduction for the IC (p = 0.001). The corresponding risk reductions were 41% and 13% for patients with NSTEMI (p = 0.003) and 45% and 9% for those with STEMI (p = 0.09). One patient with STEMI in the IC who died during transfer for primary percutaneous coronary intervention was also included in the analysis. The influence of variables predicting 1-year mortality according to univariate and multiple Cox proportional hazards regression analyses is presented in Table 2 . The IC had a 46% lower relative risk of death (hazard ratio 0.54, 95% confidence interval 0.38 to 0.78, p <0.001) after 1 year, after adjustment for age, gender, previous AMI, previous stroke, diabetes, smoking status, previous left ventricular systolic dysfunction, and serum creatinine at admission, and a 33% lower relative risk (hazard ratio 0.67, 95% confidence interval 0.46 to 0.97, p = 0.033) when adjusted for the Global Registry of Acute Coronary Events risk score. The interaction terms tested were not statistically significant.
| Variable | Univariate Cox Regression Analyses | Multiple Cox Regression Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Invasive cohort | 0.56 | 0.39–0.79 | 0.001 | 0.54 | 0.38–0.78 | 0.001 |
| Age per year | 1.07 | 1.05–1.08 | <0.001 | 1.07 | 1.05–1.09 | <0.001 |
| Male gender | 0.85 | 0.60–1.20 | 0.35 | 1.25 | 0.86–1.83 | 0.25 |
| Previous acute myocardial infarction | 2.48 | 1.76–3.49 | <0.001 | 1.48 | 0.98–2.22 | 0.064 |
| Previous stroke | 1.68 | 0.97–2.93 | 0.065 | 1.39 | 0.79–2.44 | 0.26 |
| Diabetes mellitus | 1.28 | 0.82–1.99 | 0.27 | 1.01 | 0.64–1.59 | 0.97 |
| Current smoker | 0.61 | 0.41–0.90 | 0.014 | 1.42 | 0.89–2.26 | 0.14 |
| Previous left ventricular systolic dysfunction ⁎ | 3.29 | 2.10–5.16 | <0.001 | 1.65 | 1.00–2.71 | 0.050 |
| S-creatinine (μmol/L † ) | 1.007 | 1.005–1.008 | <0.001 | 1.004 | 1.003–1.006 | <0.001 |
⁎ Defined as previous left ventricular ejection fraction <40%.
The in-hospital mortality for patients with NSTEMI not treated with early reperfusion was lower in the IC than in the CC ( Table 3 ). A nonsignificant tendency was seen for reduced postdischarge survival in the IC versus the CC. The postdischarge mortality for patients with STEMI was significantly greater in the CC (14% [16 of 117] vs 4% [4 of 99], p = 0.015). For the patients with NSTEMI, the corresponding data were 21% (34 of 159) versus 15% (29 of 191; p = 0.14).
| Variable | STEMI | NSTEMI | ||||
|---|---|---|---|---|---|---|
| CC | IC | p Value | CC | IC | p Value | |
| In-hospital deaths | ||||||
| Total cohort | 9/126 (7%) | 8/107 (7%) | 0.92 | 26/185 (14%) | 9/200 (5%) | 0.001 |
| No early reperfusion ⁎ | 4/49 (8%) | 6/37 (16%) | 0.26 | 26/177 (15%) | 8/150 (5%) | 0.006 |
| Total deaths | ||||||
| Total cohort | 25/126 (20%) | 12/107 (11%) | 0.086 | 60/185 (32%) | 38/200 (19%) | 0.002 |
| No early reperfusion ⁎ | 14/49 (29%) | 8/37 (22%) | 0.54 | 60/177 (34%) | 37/150 (25%) | 0.052 |
⁎ Defined as no primary percutaneous coronary intervention/fibrinolysis for STEMI and no percutaneous coronary intervention/coronary artery bypass grafting within 72 hours for NSTEMI.
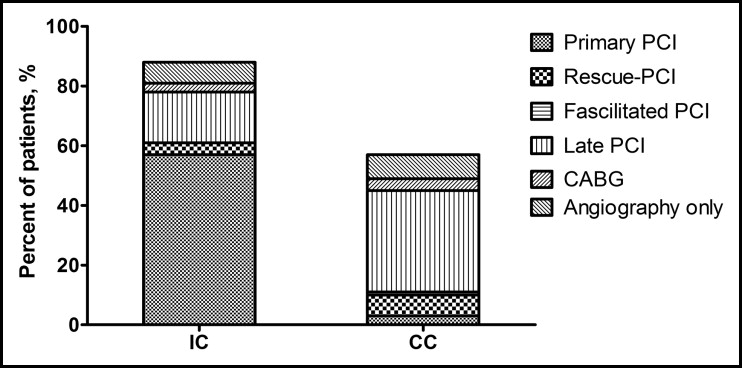
The median time from the telephone call to the emergency medical systems to arrival of an ambulance was 9 minutes and the door-to-balloon time was 20 to 30 minutes for patients with STEMI in both cohorts. Primary percutaneous coronary intervention was performed in 61 patients (57%) in the IC and 4 patients (3%) in the CC (p <0.001; Figure 2 ). The corresponding numbers for fibrinolytic therapy were 9 (8%) and 73 (58%; p <0.001). Facilitated percutaneous coronary intervention was performed in 1 patient in the CC. Rescue percutaneous coronary intervention was performed in 4 of 9 patients in the IC and 9 of 73 patients in the CC. During follow-up, another 18 patients (17%) in the IC were treated with percutaneous coronary intervention and 3 (3%) with coronary artery bypass grafting. In the CC, another 43 patients (34%) underwent percutaneous coronary intervention and 9 (8%) underwent coronary artery bypass grafting. The total revascularization rate in the IC and CC was 81% and 54%, respectively (p <0.001). Repeated percutaneous coronary intervention was performed in 10 patients in the IC and in 1 patient in the CC.