There is growing awareness that the Norwood procedure with the Sano modification is prone to early right ventricular to pulmonary artery (RV–PA) conduit stenosis resulting in systemic oxygen desaturation, increased interstage morbidity, and death. We report our experience with endovascular stent placement for conduit stenosis and compare the outcomes at stage II surgery between stented and nonstented infants. The medical records of all patients with hypoplastic left heart syndrome who received an RV–PA conduit at Norwood palliation from May 2005 to January 2010 were reviewed. The preoperative anatomy, demographics, operative variables, and outcomes pertaining to the Norwood and subsequent stage II surgeries were obtained and compared between stented and nonstented infants. The pre- and post-stent oxygen saturation, stenosis location, type and number of stents implanted, concomitant interventions, procedure-related complications, and reinterventions were collected. Of the 66 infants who underwent the Norwood procedure with RV–PA conduit modification, 16 (24%) received stents. The anatomy, demographics, and outcome variables after the Norwood procedure were similar between the stented and nonstented infants. The age at catheterization was 93 ± 48 days, and the weight was 4.9 ± 1.2 kg. The oxygen saturation increased from 66 ± 9% before intervention to 82 ± 6% immediately after stenting (p <0.0001). No interstage surgical shunt revisions were performed in either group. Age, weight, pre-stage II echocardiographic variables, oxygen saturation, and operative and outcome variables, including mortality, were similar between the 2 groups. In conclusion, endovascular stent placement for RV–PA conduit stenosis after the Norwood procedure leads to improved systemic oxygen levels and prevents early performance of stage II surgery without compromising stage II outcomes.
The mortality associated with the Norwood procedure in infants with hypoplastic left heart syndrome (HLHS) remains the greatest among the congenital heart procedures. One strategy for providing pulmonary blood flow involves the placement of a right ventricular to pulmonary artery (RV–PA) conduit instead of the traditional modified Blalock-Taussig (mBT) shunt. There is a growing awareness, however, that the RV–PA conduit is prone to early stenosis, leading to systemic oxygen desaturation and contributing to increased interstage morbidity and death. In an effort to manage this progressive cyanosis, some centers have advocate performing the stage II surgery as early as 2 months of age, and others have opted to surgically revise the RV–PA conduit or to replace it with a mBT shunt. Recently, transcatheter stent placement within the RV–PA conduit has been reported to improve systemic oxygen saturation. To date, the effects of endovascular RV–PA conduit stent implantation on the acute outcomes at the stage II surgery have not been fully investigated. Therefore, we sought to determine the risks and effectiveness of endovascular stent placement for the treatment of RV–PA conduit stenosis and to compare the stage II surgical outcomes between the infants who underwent conduit stenting and those who did not.
The institutional review boards of the University of Utah and Primary Children’s Medical Center approved this retrospective study under a waiver of informed consent. The pediatric cardiovascular surgery database was searched for all patients with HLHS who had undergone the Norwood procedure with the Sano modification from May 2005 to January 2010. The medical records were reviewed for gender, gestational age, and underlying cardiac diagnosis. The variables related to the Norwood surgery included age and weight at surgery, RV–PA conduit diameter at implantation, duration of cardiopulmonary bypass (CPB), aortic cross-clamping, circulatory arrest and days of mechanical ventilation, chest tube use, intensive care unit length of stay (LOS), hospital LOS, and discharge oxygen saturation. The interstage period was defined as the interval after Norwood discharge until admission for stage II surgery. The variables related to stage II surgery included age and weight at surgery, prestage II oxygen saturation, CPB duration, days of mechanical ventilation, chest tube use, intensive care unit LOS, and hospital LOS. All deaths were recorded up to discharge after stage II surgery.
The echocardiographic reports were reviewed for right ventricular systolic function and grade of tricuspid regurgitation before the Norwood and stage II surgeries. Right ventricular function was subjectively graded as normal or mildly, moderately, or severely decreased, and tricuspid regurgitation was graded as none/trace, mild, moderate, or severe.
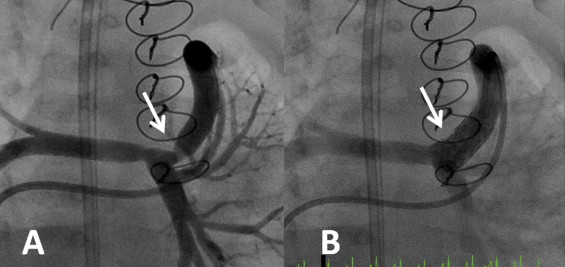
All catheterization procedures were performed with the patient under general anesthesia. A femoral venous approach was preferred whenever possible. Routinely, a 4F glide catheter (Terumo, Somerset, New Jersey) with a JB1 curve was used with a 0.035-in. glide wire to access the proximal conduit and perform manual angiography. The patients underwent stent placement if angiographic conduit narrowing was identified in the setting of concurrent severe hypoxemia ( Figure 1 ) . Initially, the stents were hand-mounted on low-profile balloons and deployed within the conduit using a long venous sheath. However, after low-profile, premounted stents became commercially available, they were used exclusively, and, in most cases, a 0.018-in. Roadrunner wire (Cook Medical, Bloomington, Indiana) was positioned in a distal branch pulmonary artery. In most cases, premounted Formula 418 stents (Cook Medical) were implanted without the use of a long guide sheath, using anatomic landmarks to guide stent placement. The stent size was based on the conduit diameter and was typically ≤1 mm larger than the conduit diameter recorded at surgical implantation and was not based on the stenotic diameter. The oxygen saturation was recorded before and after stent placement under similar hemodynamic conditions and inhaled oxygen concentrations. Resolution of conduit stenosis was confirmed on post-stent angiograms and improvement in systemic oxygen saturation. All catheterization records were reviewed for age and weight at catheterization, pre- and post-stent oxygen saturation, location of conduit stenosis, vascular access site, type of venous sheath, type and number of stents implanted, and concomitant interventions performed. All procedure-related complications and catheter-based reinterventions were noted.
The patients were divided into 2 groups: those with endovascular stent implantation, and those without stenting of the conduit. The variables collected from both groups at all designated intervals were analyzed. Comparisons between groups and independent predictors of stent placement were made using the Wilcoxon rank sum tests, Fisher exact tests, or chi-square tests, as appropriate. Multivariate logistic regression analysis was performed to identify the effects of anatomic subtype on overall mortality. Data are expressed as mean ± SD, unless otherwise specified. p Values of <0.05 were considered significant.
During the study period, 66 (86%) of 77 infants with HLHS, or a related variant, underwent the Norwood procedure with Sano modification and formed the study group (7 patients with mBT shunt, and 4 patients with bilateral PA bands and ductal stents were excluded). Of these 66 infants with RV–PA conduits, 16 (24%) received stents. The cardiac anatomy, demographics, initial echocardiographic findings, and outcome variables after Norwood palliation were similar between those with and without conduit stents ( Table 1 ). The potential risk factors for conduit stenting were investigated. Gestational age, birth weight, age at Norwood, anatomic subtype, RV–PA conduit diameter, pre-Norwood RV systolic function and tricuspid valve regurgitation grade, CPB duration, aortic cross-clamping duration, circulatory arrest and days of mechanical ventilation, chest tube use, intensive care unit LOS, hospital LOS, and post-Norwood discharge oxygen saturation were entered into the univariate model. None of these variables was associated with using conduit stents.
| Variable | Conduit Stent (n = 16) | No Conduit Stent (n = 50) | p Value |
|---|---|---|---|
| Anatomic subtype | 0.22 | ||
| Aortic atresia/mitral atresia | 6 | 17 | |
| Aortic stenosis/mitral stenosis | 6 | 16 | |
| Aortic atresia/mitral stenosis | 1 | 13 | |
| Aortic stenosis/mitral atresia | 2 | 1 | |
| Hypoplastic left heart syndrome variant ⁎ | 1 | 3 | |
| Gestational age (weeks) | 38.1 ± 1.8 | 38.1 ± 1.3 | 0.51 |
| Age at Norwood (days) | 7.4 ± 4.0 | 5.8 ± 2.0 | 0.34 |
| Birth weight (kg) | 3.2 ± 0.6 | 3.0 ± 0.5 | 0.17 |
| Echocardiographic systolic function (n) | |||
| Normal | 15 | 44 | 1.00 |
| Mildly decreased | 1 | 5 | |
| Moderately decreased | 0 | 1 | |
| Severely decreased | 0 | 0 | |
| Echocardiographic atrioventricular valve regurgitation (n) | |||
| None/trace | 11 | 34 | 0.96 |
| Mild | 5 | 16 | |
| Moderate | 0 | 0 | |
| Severe | 0 | 0 | |
| Right ventricular–pulmonary artery conduit diameter † (mm) | 5.2 ± 0.4 | 5.0 ± 0.3 | 0.06 |
| Cardiopulmonary bypass time (minutes) | 144 ± 25 | 156 ± 48 | 0.63 |
| Aortic cross-clamp (minutes) | 57 ± 12 | 55 ± 14 | 0.50 |
| Circulatory arrest (minutes) | 6 ± 3 | 6 ± 5 | 0.15 |
| Mechanical ventilation (days) | 20 ± 18 | 16 ± 19 | 0.31 |
| Chest tube use (days) | 25 ± 18 | 19 ± 19 | 0.09 |
| Intensive care unit stay (days) | 32 ± 23 | 24 ± 22 | 0.10 |
| Total hospital stay (days) | 42 ± 24 | 31 ± 22 | 0.09 |
| Discharge oxygen saturation (%) | 78 ± 5 | 78 ± 4 | 0.99 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


