An elevated heart rate (HR) at rest at baseline is associated with an increased risk of incident heart failure (HF) and with greater cardiovascular event rates in patients with chronic HF. However, despite the high attributable risk of hypertension for HF, whether the in-treatment HR predicts incident HF in patients with treated hypertension has not been evaluated. The HR was evaluated on annual electrocardiograms from 9,024 patients with hypertension without HF who were treated with losartan- or atenolol-based regimens. During a mean follow-up of 4.7 ± 1.1 years, HF developed in 285 patients (3.2%). On multivariate Cox analyses adjusted for randomized treatment, the baseline risk factors for HF, baseline and in-treatment blood pressure, QRS duration, and electrocardiographic left ventricular hypertrophy, a greater in-treatment HR predicted a 45% greater adjusted risk of new HF for every 10-beats/min increase in the HR (95% confidence interval [CI] 34% to 57%) or a 159% greater risk of HF in patients with the persistence or development of a HR of ≥84 beats/min (95% CI 88% to 257%). In contrast, with adjustment for the same covariates, the baseline HR as a continuous variable was a significantly less powerful predictor of new HF (hazard ratio 1.15 per 10 beats/min, 95% CI 1.03 to 1.28) and a baseline HR of ≥84 beats/min did not predict new HF (hazard ratio 1.00, 95% CI 0.63 to 1.58). In conclusion, a greater in-treatment HR on the serial electrocardiograms predicts a greater risk of incident HF during antihypertensive treatment, independent of the covariates, in patients with hypertension with electrocardiographic left ventricular hypertrophy. These findings support serial HR assessment to improve the risk stratification of patients with hypertension.
A high heart rate (HR) at rest has been proposed as a potential simple marker of risk and an increased risk of new or recurrent heart failure (HF). Reducing the HR using the selective I (f) channel inhibitor, ivabradine, significantly reduced the composite end point of cardiovascular death and hospitalization for worsening HF in patients with chronic HF but did not reduce hospitalization for new or worsening HF in patients with stable coronary disease and left ventricular (LV) dysfunction at greater risk owing to a baseline HR of ≥70 beats/min. Although a greater in-treatment HR over time has been associated with an increased risk of cardiovascular and all-cause mortality and incident atrial fibrillation in patients with hypertension, the relation of in-treatment HR over time to incident HF has not been evaluated. Therefore, the present study examined whether a greater HR over time is associated with an increased risk of HF in patients with hypertension undergoing treatment, independent of the effects of in-treatment blood pressure and other risk factors for HF and of the previously demonstrated relation of in-treatment electrocardiographic LV hypertrophy (LVH) and QRS duration to incident HF.
Methods
The Losartan Intervention For Endpoint reduction (LIFE) study enrolled 9,193 patients with hypertension with electrocardiographic LVH using the Cornell voltage-duration product and/or Sokolow-Lyon voltage criteria on a screening electrocardiogram in a prospective, double-blind randomized study that compared cardiovascular morbidity and mortality with the use of losartan- versus atenolol-based treatment, as previously described in detail. A total of 166 patients with a history of HF and 3 with missing baseline HR data were excluded from the analyses, leaving 9,024 patients in the present study. Blinded treatment was begun with losartan 50 mg/day or atenolol 50 mg/day and matching placebo of the other agent, with uptitration of the study medication and the addition of additional nonstudy medications (hydrochlorothiazide, calcium channel blocker, or other medications excluding angiotensin II receptor type 1 or β blockers or angiotensin-converting enzyme inhibitors) to achieve a target pressure of ≤140/90 mm Hg, as previously reported in detail. Nonstudy medication use did not differ between the treatment arms.
Study electrocardiograms were obtained at baseline, at 6 months of follow-up and then yearly until study termination or patient death and were interpreted as previously reported in detail. A Cornell product >2,440 mm · ms or Sokolow-Lyon voltage >38 mm was used to identify LVH. The HR was measured to the nearest beats per minute on each protocol-mandated study electrocardiogram.
Hospitalization for HF was a prespecified secondary end point in the LIFE study, with the diagnosis of HF determined from the clinical and diagnostic findings. Each case was reviewed and verified by the end point committee who were unaware of the study electrocardiographic LVH findings when classifying the possible morbid events.
Data management and analyses were performed by the investigators using the Statistical Package for Social Sciences, version 12.0 (SPSS, Chicago, Illinois). The data are presented as the mean ± SD for continuous variables and proportions for categorical variables. Differences in the mean values between patients grouped according to the baseline HR partitioned at 84 beats/min (the upper quintile of the baseline HR in this population and a value previously shown to stratify risk) were compared using unpaired t tests. A comparison of the proportions between groups was performed using chi-square tests.
The relation of HR on baseline and in-study electrocardiograms to risk of incident HF was assessed using Cox proportional hazards models. The baseline risk factors, a treatment group indicator, and the baseline HR, QRS duration, systolic and diastolic pressure, Cornell product, and Sokolow-Lyon voltage were included as standard covariates, and the subsequent in-treatment blood pressure, HR, QRS duration, Cornell product, and Sokolow-Lyon voltage measurements were entered as time-varying covariates. In addition, the relation of the persistence or development of a HR ≥84 versus a HR <84 beats/min treated as a dichotomous time-varying variable to incident HF was also analyzed. Hazard ratios for new HF associated with in-treatment HR treated as a continuous variable were computed for each 10-beats/min greater HR values. The analyses were repeated, stratifying the population by relevant subgroups by adding cross-product terms of time-varying HR and these subgroup variables into models in the total population.
To illustrate the results of the time-varying covariate analyses, the HF hospitalization rates over time were plotted as a function of the changing presence or absence of a HR of ≥84 beats/min using a univariate modified Kaplan-Meier method, implemented in SAS, release 8.2 on the WIN_PRO platform (SAS Institute, Cary, North Carolina). Two-tailed p <0.05 was required for statistical significance.
The trial was registered with clinicaltrials.gov (registration number NCT00338260 ).
Results
The clinical and demographic characteristics of patients in relation to the baseline HR partitioned at 84 beats/min are listed in Table 1 . Patients with hypertension with a baseline HR of ≥84 beats/min were older, were more likely to be women and not black, to have diabetes, and to be current smokers. They had a greater body mass index, serum glucose, and total cholesterol levels and greater albuminuria, but were similar with respect to treatment randomization and other baseline characteristics.
| Variable | HR (beats/min) | p Value | |
|---|---|---|---|
| <84 (n = 7,199) | ≥84 (n = 1825) | ||
| Age (years) | 66.7 ± 7.0 | 67.5 ± 6.9 | <0.001 |
| Women | 51.6% | 63.3% | <0.001 |
| Black | 6.0% | 4.7% | 0.037 |
| Randomized to losartan | 50.3% | 49.1% | 0.361 |
| Diabetes | 11.9% | 15.7% | <0.001 |
| Previous coronary heart disease ⁎ | 15.2% | 15.4% | 0.575 |
| Previous myocardial infarction | 6.1% | 6.6% | 0.825 |
| Previous stroke | 4.3% | 4.1% | 0.74 |
| Previous peripheral vascular disease | 5.6% | 5.3% | 0.712 |
| Previous atrial fibrillation | 3.0% | 4.1% | 0.025 |
| Current smoker | 15.6% | 19.7% | <0.001 |
| Body mass index (kg/m 2 ) | 27.9 ± 4.6 | 28.2 ± 5.2 | 0.01 |
| Serum glucose (mmol/L) | 5.90 ± 2.07 | 6.46 ± 2.51 | <0.001 |
| Serum creatinine (mmol/L) | 86.9 ± 20.1 | 85.9 ± 19.3 | 0.074 |
| Total cholesterol | 0.001 | ||
| mmol/L | 6.03 ± 1.11 | 6.12 ± 1.16 | |
| mg/dl | 233 ± 43 | 236 ± 45 | |
| High-density lipoprotein cholesterol | 0.32 | ||
| mmol/L | 1.49 ± 0.44 | 1.51 ± 0.44 | |
| mg/dl | 58 ± 17 | 58 ± 17 | |
| Urine albumin/creatinine ratio (mg/mM) | 6.6 ± 27.4 | 9.9 ± 48.2 | <0.001 |
⁎ Previous coronary heart disease was defined as clinical history of coronary disease in absence of myocardial infarction within previous 6 months or presence of angina pectoris requiring treatment with β blocker or calcium antagonist, because these were exclusions from the Losartan Intervention For Endpoint reduction (LIFE) study.
The blood pressure and electrocardiographic LVH measurements at baseline and the changes in these measurements between the baseline and last in-study determination or the last measurement before hospitalization for HF in relation to the HR at baseline are listed in Table 2 . Patients with a baseline HR of ≥84 beats/min had slightly greater baseline systolic and diastolic pressures and a greater reduction in diastolic pressure but a similar change in systolic pressure. Patients with a baseline HR of ≥84 beats/min had less severe LVH using the Sokolow-Lyon voltage and a shorter QRS duration at baseline and greater increases in the QRS duration during the study but a similar baseline severity of the Cornell product LVH and similar changes in both electrocardiographic LVH criteria.
| Variable | HR (beats/min) | p Value | |
|---|---|---|---|
| <84 (n = 7,199) | ≥84 (n = 1,825) | ||
| Baseline measurements | |||
| Systolic blood pressure (mm Hg) | 174 ± 14 | 175 ± 15 | 0.009 |
| Diastolic blood pressure (mm Hg) | 97 ± 9 | 100 ± 9 | <0.001 |
| Cornell voltage-duration product (mm · ms) | 2,805 ± 1,015 | 2,846 ± 1,034 | 0.128 |
| Sokolow-Lyon voltage (mm) | 30.2 ± 10.4 | 28.9 ± 10.2 | <0.001 |
| QRS duration (ms) | 101.5 ± 17.9 | 99.4 ± 18.3 | <0.001 |
| Change from baseline to last measurement ⁎ | |||
| Systolic blood pressure (mm Hg) | −30 ± 20 | −30 ± 20 | 0.616 |
| Diastolic blood pressure (mm Hg) | −17 ± 10 | −18 ± 11 | <0.001 |
| Cornell voltage-duration product (mm · ms) | −192 ± 855 | −222 ± 827 | 0.174 |
| Sokolow-Lyon voltage (mm) | −3.9 ± 7.3 | −3.6 ± 7.3 | 0.082 |
| QRS duration (ms) | 1.5 ± 12.2 | 2.6 ± 12.6 | 0.001 |
⁎ Change from baseline to last in-study measurement or last measurement before death.
During a mean follow-up of 4.7 ± 1.1 years, HF developed in 285 patients (3.2%). Compared to the patients who did not develop HF, those who did develop HF had a significantly greater HR on the last electrocardiogram before new HF or the last in-treatment electrocardiogram (74.9 ± 13.6 vs 68.6 ± 11.7 beats/min), with similar mean differences with losartan-based (5.7 beats/min) or atenolol-based (7.0 beats/min, all p <0.001) treatment. New-onset HF occurred in 67 patients with in-treatment persistence or the development of a HR of ≥84 beats/min, a rate of 13.3/1,000 patient-years, and in 218 patients with in-treatment development or the continued presence of a HR <84 beats/min, a rate of 5.6/1,000 patient-years.
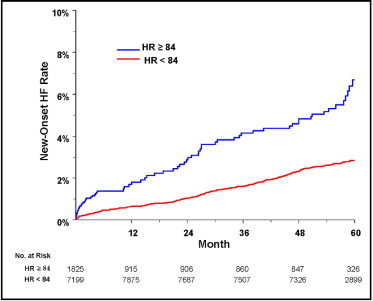
On univariate Cox analyses in which the time-varying HR was treated as a continuous variable ( Table 3 ), a higher in-treatment HR was strongly associated with an increased risk of developing HF: every 10-beats/min greater HR was associated with a 42% increased risk of new HF. In parallel analyses in which in-treatment HR was treated as a dichotomous variable with a threshold value of ≥84 beats/min, the in-treatment persistence or development of a HR of ≥84 beats/min was associated with a 166% greater risk of developing HF compared to the development or persistence of a HR of <84 beats/min. Modified Kaplan-Meier curves comparing the rate of new HF according to a HR of 84 beats/min during the study period ( Figure 1 ) demonstrated that the persistence or development of a HR of ≥84 beats/min was associated with a greater risk of developing HF compared to a HR of <84 beats/min, with the persistence or development of a HR of ≥84 beats/min associated with an estimated 2.2% greater absolute incidence of new HF after 4 years of follow-up.
| Predictor Variable | p Value | Hazard Ratio | 95% CI |
|---|---|---|---|
| Univariate | |||
| Heart rate (per 10 beats/min increase) | <0.001 | 1.42 | 1.31–1.54 |
| Heart rate (persistence or development of HR ≥84 beats/min) ⁎ | <0.001 | 2.66 | 2.02–3.49 |
| Multivariate † | |||
| Heart rate (per 10 beats/min increase) | <0.001 | 1.45 | 1.34–1.57 |
| Heart rate (persistence or development of HR ≥84 beats/min) ⁎ | <0.001 | 2.59 | 1.88–3.57 |
⁎ New-onset HF occurred in 67 patients with in-treatment persistence or development of HR ≥84 beats/min, a rate of 13.3/1,000 patient-years, and in 218 patients with in-treatment development or continued presence of HR <84 beats/min, a rate of 5.6/1,000 patient-years.
† Adjusted for possible effects of treatment with losartan versus atenolol, age, gender, race, prevalent diabetes, history of ischemic heart disease, myocardial infarction, atrial fibrillation, stroke, peripheral vascular disease or smoking, baseline HR, albumin/creatinine ratio, total and high-density lipoprotein cholesterol, serum creatinine, serum glucose, body mass index, baseline and in-treatment systolic and diastolic blood pressure, QRS duration, Sokolow-Lyon voltage, and Cornell voltage-duration product.
The relation of new-onset HF to in-treatment HR was further examined after adjusting for the possible effects of randomized treatment, age, gender, race, prevalent diabetes, a history of ischemic heart disease, myocardial infarction, stroke, peripheral vascular disease and smoking, prevalent atrial fibrillation by history or baseline electrocardiogram, baseline HR, urinary albumin/creatinine ratio, total and high-density lipoprotein cholesterol, serum creatinine, body mass index, and for baseline and in-treatment systolic and diastolic blood pressure, the QRS duration, Cornell product and Sokolow-Lyon voltage ( Table 3 ). After adjusting for these factors, every 10-beats/min higher in-treatment HR was associated with a 45% greater risk of new HF. In parallel analyses, in-treatment persistence or the development of a HR of ≥84 beats/min remained associated with a 159% increased adjusted risk of developing new HF. In contrast, the baseline HR was a significantly less powerful predictor of new HF when adjusted for the same covariates: every 10-beats/min greater baseline HR was associated with a 15% increased risk (hazard ratio 1.15, 95% confidence interval 1.03 to 1.28). In contrast, a baseline HR of ≥84 beats/min was not associated with even a trend toward an increased risk of incident HF (hazard ratio 1.00, 95% confidence interval 0.63 to 1.58).
The predictive value of the time-varying HR for incident HF in the relevant subsets of the population is examined in Table 4 . The association of new HF with in-treatment HR was similar in patients grouped according to race, treatment allocation, age ≥65 years, a history of ischemic heart disease or myocardial infarction, prevalent diabetes, prevalent or a history of atrial fibrillation, the baseline presence or absence of electrocardiographic LVH by Cornell product and Sokolow-Lyon criteria, and the baseline HR partitioned at a HR of 84 beats/min. In contrast, women had a significantly steeper increase in the risk of new HF with a greater in-treatment HR than men.
| Subgroup | New HF (n) | Hazard Ratio ⁎ | 95% CI | p Value for Interaction † |
|---|---|---|---|---|
| Gender | 0.031 | |||
| Male (n = 4,151) | 143 | 1.35 | 1.20–1.52 | |
| Female (n = 4,873) | 142 | 1.68 | 1.44–1.93 | |
| Race | 0.224 | |||
| White or other (n = 8,510) | 256 | 1.42 | 1.31–1.54 | |
| Black (n = 514) | 29 | 1.23 | 0.80–1.91 | |
| Treatment | 0.391 | |||
| Atenolol (n = 4,505) | 146 | 1.41 | 1.27–1.58 | |
| Losartan (n = 4,519) | 139 | 1.45 | 1.27–1.68 | |
| Age (years) | 0.188 | |||
| <65 (n = 3,453) | 59 | 1.61 | 1.37–1.91 | |
| ≥65 (n = 5,571) | 226 | 1.37 | 1.24–1.52 | |
| History of coronary heart disease | 0.45 | |||
| No (n = 7,652) | 181 | 1.41 | 1.27–1.57 | |
| Yes (n = 1,372) | 104 | 1.42 | 1.21–1.68 | |
| History of myocardial infarction | 0.217 | |||
| No (n = 8,508) | 232 | 1.44 | 1.32–1.57 | |
| Yes (n = 516) | 53 | 1.58 | 1.15–2.16 | |
| Diabetes mellitus | 0.783 | |||
| No (n = 7,877) | 209 | 1.41 | 1.29–1.55 | |
| Yes (n = 1,147) | 76 | 1.45 | 1.18–1.81 | |
| Atrial fibrillation by history or on baseline electrocardiogram | 0.675 | |||
| No (n = 8,699) | 246 | 1.4 | 1.28–1.52 | |
| Yes (n = 325) | 39 | 1.81 | 1.29–2.52 | |
| Cornell product LVH on baseline electrocardiogram | 0.721 | |||
| No (n = 2,984) | 73 | 1.48 | 1.23–1.79 | |
| Yes (n = 6,040) | 212 | 1.44 | 1.31–1.58 | |
| Sokolow-Lyon voltage left ventricular hypertrophy on baseline electrocardiogram | 0.279 | |||
| No (n = 7,116) | 210 | 1.45 | 1.32–1.60 | |
| Yes (n = 1,908) | 75 | 1.31 | 1.06–1.61 | |
| Heart rate on baseline electrocardiogram | 0.279 | |||
| <84 beats/min (n = 7,199) | 207 | 1.38 | 1.26–1.52 | |
| ≥84 beats/min (n = 1,825) | 78 | 1.63 | 1.33–2.02 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


