For patients at high cardiovascular and high gastrointestinal (GI) risk, coprescription of a proton pump inhibitor (PPI) with low-dose aspirin is recommended. We aimed to quantify the extent to which low-dose aspirin discontinuation in patients at high cardiovascular risk is affected by PPI use and baseline GI risk. Patients aged 50 to 84 years who had evidence of ischemic heart disease or cardiovascular disease and who were new users of low-dose aspirin in 2000 to 2007 were identified using The Health Improvement Network (n = 35,604). Aspirin discontinuation was defined as a period of at least 90 days after completion of the last prescribed course during which no repeat prescription was issued. The incidence of low-dose aspirin discontinuation was 26.8 per 100 person-years (95% confidence interval [CI] 26.2 to 27.4). The age-, gender-, and indication-adjusted risk of aspirin discontinuation was 15% less among continuous PPI users than among PPI nonusers (hazard ratio [HR] 0.85, 95% CI 0.78 to 0.92); after further adjusting for number of coprescribed medications, the HR was 0.95 (95% CI 0.87 to 1.03). Continuous PPI use was associated with a reduced risk of aspirin discontinuation among patients at high GI risk (HR 0.83; 95% CI 0.74 to 0.93) but not among those at low GI risk (HR 1.08; 95% CI 0.96 to 1.21). In conclusion, among patients at high GI risk, concomitant users of aspirin and PPI showed a greater aspirin adherence than aspirin users not on PPI. Further studies need to confirm factors with the potential to increase adherence to long-term aspirin.
Low-dose aspirin is widely prescribed for the secondary prevention of ischemic heart disease (IHD) and cerebrovascular disease. At-risk patients need to continue with long-term aspirin as discontinuation is associated with an increased risk of ischemic coronary and cerebrovascular events. Subjects who experience adverse gastrointestinal (GI) complications and symptoms are at particular risk of discontinuing their aspirin. Discontinuation may, thus, vary according to whether patients have a high or low GI risk profile and whether high risk is adequately managed. For patients who require low-dose aspirin because of cardiovascular (CV) risk and are at high GI risk, expert consensus recommends coprescription of a proton pump inhibitor (PPI) to minimize aspirin-associated GI complications. Use of PPIs in patients at high CV risk and high GI risk could, thus, increase adherence to aspirin, thereby helping to maintain CV protection. Limited data exist on whether PPI use increases adherence to low-dose aspirin in these patients. We aimed to quantify the extent to which discontinuation of low-dose aspirin, prescribed to patients for secondary CV event prevention, is affected by PPI use and by whether patients have a high or low baseline GI risk profile.
Methods
This was a pharmacoepidemiology study that used data from The Health Improvement Network (THIN) primary care database in the United Kingdom. Patients aged 50 to 84 years with evidence of IHD or cerebrovascular disease who were new users of low-dose aspirin (75 to 300 mg/day) and who had received at least 2 low-dose aspirin prescriptions for the secondary prevention of CV disease (myocardial infarction, angina, or unspecified IHD) or cerebrovascular disease (stroke, transient ischemic attack, or unspecified cerebrovascular disease) from 2000 to 2007 were identified from THIN. Patients were required to have been enrolled with their primary care practitioner (PCP) for at least 2 years and to have a computerized prescription history of at least 1 year before the start of the study. Patients were excluded if they had received a diagnosis of cancer, alcohol abuse, or alcohol-related disease, or if data recording in THIN was incomplete. Ethical approval for the study was obtained from a Multicentre Research Ethics Committee (National Health Service; MREC reference number: 08/H0305/49).
THIN contains anonymized computerized information on >3 million patients, which is entered by PCPs. The recorded data include patient demographics, diagnoses and symptoms (recorded using Read codes ), details of specialist referrals and hospital admissions, and results of laboratory tests. Prescriptions issued by the PCP are generated from their computer and so are automatically recorded. THIN covers about 5% of the United Kingdom population and is representative of the general population in terms of age, gender, and geographical distribution. Validation studies have shown THIN to be appropriate for use in pharmacoepidemiology research.
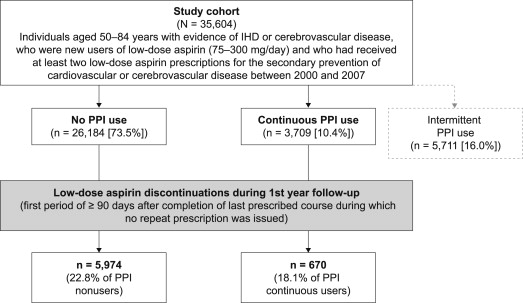
Patients were followed up for up to 1 year from the day of their first low-dose aspirin prescription (start date) until the earliest of the following: date of low-dose aspirin discontinuation, a diagnosis of cancer or alcohol-related condition, death, or the end of the study period (December 31, 2007). Discontinuation of low-dose aspirin was defined as the first period of at least 90 days after completion of the last prescribed course of low-dose aspirin during which no repeat prescription was issued.
For each patient, PPI use was ascertained from the database and was considered as being continuous if the patient was exposed at the start date and throughout their follow-up period. Patients with gaps in their PPI therapy ≤30 days were still deemed to be continuous PPI users. PPI nonuse was defined as no PPI use at any time during a patient’s individual follow-up period. Patients who did not fall into either category were considered to be intermittent users and were not included in the present analysis. We also evaluated the association between prescription of PPI at the start date and factors that could potentially be confounders of the effect of PPI on aspirin discontinuation.
Patients were classed as being at either high or low baseline GI risk on the basis of the presence or absence of established GI risk factors (according to expert consensus ) at the start date. Subjects were considered to be at high GI risk if they had a record of ≥1 of the following: a history of ulcer complications, including a peptic ulcer bleed (any time before the start date); a history of uncomplicated symptomatic peptic ulcer (any time before the start date); concomitant use of nonsteroidal anti-inflammatory drugs (≥1 prescription in 30 days after the start date); concomitant use of oral anticoagulant therapy (≥1 warfarin prescription in 30 days after the start date); concomitant use of antiplatelet therapy (clopidogrel or any other antiplatelet therapy other than low-dose aspirin; ≥1 prescription in 30 days after the start date); a positive test result for Helicobacter pylori , with no recorded eradication treatment after the positive test result; eradication treatment for H pylori (in the month before the start date or at any time after the start date); or at least 2 of the following risk factors: age ≥60 years, concomitant use of oral or injected corticosteroid therapy (≥1 prescription in 30 days after the start date), diagnosis of dyspepsia, gastroesophageal reflux disease, heartburn, regurgitation, or epigastric pain (in the year before the start date). Patients not qualifying as being at high GI risk were considered to be at low GI risk.
The incidence and 95% confidence interval (CI) of low-dose aspirin discontinuation per 100 person-years was calculated in the entire study cohort. Crude logistic regression analysis (odds ratio and 95% CI) was carried out to estimate the association between potential confounders of the effect of continuous PPI use on aspirin discontinuation and continuous PPI use. Kaplan-Meier survivor functions were performed to evaluate the time to discontinuation according to PPI exposure category and baseline GI risk category. Cox regression analyses (hazard ratio and 95% CI) with adjustment for potential confounders were carried out to estimate the risk of low-dose aspirin discontinuation associated with continuous PPI use (vs PPI nonuse) and baseline GI risk category (high vs low risk). Information on confounding variables (lifestyle factors, morbidity, and comedications) was collected before the start date.
Results
A total of 35,604 patients aged 50 to 84 years who had evidence of IHD or cerebrovascular disease and who were new users of low-dose aspirin during the study period were identified. Patient characteristics of the study cohort have been described previously. During a mean follow-up of 9.8 months, 7,636 patients (21.4%) had an episode of discontinuation with low-dose aspirin, corresponding to an incidence of discontinuation of 26.8 per 100 person-years (95% CI 26.2 to 27.4). Among patients discontinuing with aspirin, the median time to discontinuation after the first prescription of low-dose aspirin was 3.7 months.
Overall, 3,709 patients were continuous PPI users and 26,184 were PPI nonusers ( Figure 1 ). The characteristics of patients who were continuous PPI users and those who were PPI nonusers are listed in Table 1 . In total, 13,617 patients were classed as being at high GI risk and 21,947 patients as being at low GI risk at baseline. Of those classed as being at high risk, 80.2% were aged ≥60 years, 12.6% had a record of GI symptoms, 8.2% had a recorded positive test result for H pylori , 5.9% and 14.1% had received diagnoses of complicated and uncomplicated peptic ulcer, respectively, and concomitant nonsteroidal anti-inflammatory drug, warfarin, or antiplatelet therapy was received by 30.4%, 6.4%, and 37.9%, respectively.
| Variable | Continuous PPI Use, n = 3,709 (%) | PPI Nonuse, n = 26,184 (%) | Unadjusted OR ∗ (95% CI) |
|---|---|---|---|
| Women | 1,801 (48.6) | 11,149 (42.6) | 1.27 (1.19–1.36) |
| Age at first aspirin prescription (yrs) | |||
| 50–59 | 668 (18.0) | 5,253 (20.1) | 1.0 |
| 60–69 | 1,156 (31.2) | 8,958 (34.2) | 1.01 (0.92–1.12) |
| 70–79 | 1,343 (36.2) | 8,985 (34.3) | 1.18 (1.06–1.30) |
| 80–84 | 542 (14.6) | 2,988 (11.4) | 1.43 (1.26–1.61) |
| Aspirin indication | |||
| Cerebrovascular disease | 983 (26.5) | 8,999 (34.4) | 0.55 (0.50–0.60) |
| IHD | 1,512 (40.8) | 11,093 (42.4) | 0.68 (0.63–0.74) |
| Unstable angina | 82 (2.2) | 442 (1.7) | 0.93 (0.73–1.18) |
| Myocardial infarction | 1,132 (30.5) | 5,650 (21.6) | 1.0 |
| Body mass index (kg/m 2 ) | |||
| 13–19 | 105 (2.8) | 674 (2.6) | 1.24 (1.00–1.54) |
| 20–24 | 797 (21.5) | 6,340 (24.2) | 1.0 |
| 25–29 | 1,343 (36.2) | 9,394 (35.9) | 1.14 (1.04–1.25) |
| ≥30 | 904 (24.4) | 5,497 (21.0) | 1.31 (1.18–1.45) |
| Unknown | 560 (15.1) | 4,279 (16.3) | 1.04 (0.93–1.17) |
| Smoker | |||
| Never | 1,491 (40.2) | 11,128 (42.5) | 1.0 |
| Present | 586 (15.8) | 5,172 (19.8) | 0.85 (0.76–0.94) |
| Former | 1,479 (39.9) | 8,291 (31.7) | 1.33 (1.23–1.44) |
| Unknown | 153 (4.1) | 1,593 (6.1) | 0.72 (0.60–0.85) |
| Number of concomitant medications † | |||
| 0–2 | 509 (13.7) | 8,990 (34.3) | 1.0 |
| 3–5 | 1,372 (37.0) | 12,036 (46.0) | 2.01 (1.81–2.24) |
| 6–10 | 1,537 (41.4) | 4,795 (18.3) | 5.66 (5.09–6.30) |
| ≥11 | 291 (7.9) | 363 (1.4) | 14.16 (11.85–16.92) |
| Number of PCP visits ‡ | |||
| 0–5 | 422 (11.4) | 6,261 (23.9) | 1.0 |
| 6–10 | 909 (24.5) | 8,328 (31.8) | 1.62 (1.44–1.83) |
| ≥11 | 2,378 (64.1) | 11,595 (44.3) | 3.04 (2.73–3.39) |
| Referrals ‡ | 3,036 (81.9) | 18,005 (68.8) | 2.05 (1.88–2.24) |
| Hospitalizations ‡ | 1,534 (41.4) | 6,905 (26.4) | 1.97 (1.83–2.11) |
∗ OR for continuous PPI use in patients with the relevant characteristic.
† Number of concomitant medications (not including PPIs) during the month before the first aspirin prescription.
The patient characteristics of low-dose aspirin discontinuers and low-dose aspirin nondiscontinuers are listed in Table 2 . Comedications used in the week before and at the time of the patients’ first aspirin prescription are listed in Table 3 . Similar associations were observed when comedication use during the first month after initiation of low-dose aspirin was assessed (data not shown).
| Variable | Aspirin Discontinuers, n = 7,636 (%) | Aspirin Nondiscontinuers, n = 27,968 (%) | HR ∗ (95% CI) |
|---|---|---|---|
| Women | 3,452 (45.2) | 12,211 (43.7) | 1.05 (1.00–1.10) |
| Age at first aspirin prescription (yrs) | |||
| 50–59 | 2,105 (27.6) | 4,931 (17.6) | 1.0 |
| 60–69 | 2,339 (30.6) | 9,796 (35.0) | 0.57 (0.54–0.61) |
| 70–79 | 2,423 (31.7) | 9,818 (35.1) | 0.58 (0.55–0.62) |
| 80–84 | 769 (10.1) | 3,423 (12.2) | 0.58 (0.53–0.63) |
| Aspirin indication | |||
| Cerebrovascular disease | 2,502 (32.8) | 9,069 (32.4) | 1.64 (1.53–1.76) |
| IHD | 3,750 (49.1) | 11,251 (40.2) | 1.84 (1.72–1.96) |
| Unstable angina | 148 (1.9) | 530 (1.9) | 1.52 (1.28–1.81) |
| Myocardial infarction | 1,236 (16.2) | 7,118 (25.5) | 1.0 |
| Body mass index (kg/m 2 ) | |||
| 13–19 | 206 (2.7) | 735 (2.6) | 1.06 (0.92–1.23) |
| 20–24 | 1,889 (24.7) | 6,692 (23.9) | 1.0 |
| 25–29 | 2,765 (36.2) | 10,055 (36.0) | 0.96 (0.90–1.02) |
| ≥30 | 1,614 (21.1) | 6,037 (21.6) | 0.88 (0.82–0.94) |
| Unknown | 1,162 (15.2) | 4,449 (15.9) | 0.94 (0.88–1.01) |
| Smoker | |||
| Never | 3,194 (41.8) | 11,727 (41.9) | 1.0 |
| Present | 1,625 (21.3) | 5,226 (18.7) | 1.09 (1.02–1.16) |
| Former | 2,448 (32.1) | 9,377 (33.5) | 0.99 (0.94–1.04) |
| Unknown | 369 (4.8) | 1,638 (5.9) | 0.87 (0.78–0.96) |
| Number of concomitant medications † | |||
| 0–2 | 2,949 (38.6) | 7,395 (26.4) | 1.0 |
| 3–5 | 3,114 (40.8) | 12,608 (45.1) | 0.65 (0.62–0.68) |
| 6–10 | 1,417 (18.6) | 7,079 (25.3) | 0.56 (0.52–0.60) |
| ≥11 | 156 (2.0) | 886 (3.2) | 0.50 (0.43–0.59) |
| Number of PCP visits ‡ | |||
| 0–5 | 1,665 (21.8) | 5,828 (20.8) | 1.0 |
| 6–10 | 2,337 (30.6) | 8,419 (30.1) | 0.97 (0.91–1.03) |
| ≥11 | 3,634 (47.6) | 13,721 (49.1) | 0.92 (0.87–0.98) |
| Referrals ‡ | 5,362 (70.2) | 20,265 (72.5) | 0.91 (0.87–0.96) |
| Hospitalizations ‡ | 1,790 (23.4) | 8,819 (31.5) | 0.77 (0.73–0.81) |
| High GI risk profile § | 2,612 (34.2) | 11,005 (39.4) | 0.89 (0.85–0.93) |
| Peptic ulcer disease ¶ | 447 (5.9) | 1,506 (5.4) | 1.11 (1.01–1.22) |
| GERD ¶ | 1,174 (15.4) | 4,090 (14.6) | 1.03 (0.96–1.09) |
| Epigastric pain ¶ | 665 (8.7) | 2,089 (7.5) | 1.12 (1.03–1.21) |
| Dyspepsia and gastritis ¶ | 1,691 (22.1) | 5,663 (20.2) | 1.08 (1.02–1.14) |
| Hypertension ¶ | 3,750 (49.1) | 14,679 (52.5) | 0.86 (0.82–0.90) |
| Atrial fibrillation ¶ | 502 (6.6) | 1,420 (5.1) | 1.38 (1.26–1.51) |
| Anemia ¶ | 511 (6.7) | 1,638 (5.9) | 1.14 (1.04–1.24) |
| Osteoarthritis ¶ | 2,658 (34.8) | 9,861 (35.3) | 1.01 (0.96–1.06) |
| Rheumatoid arthritis ¶ | 207 (2.7) | 769 (2.7) | 1.01 (0.88–1.16) |
| Heart failure ¶ | 367 (4.8) | 1,588 (5.7) | 0.95 (0.86–1.06) |
| Diabetes ¶ | 896 (11.7) | 3,715 (13.3) | 0.86 (0.80–0.92) |
| Surgery ¶ | 5,315 (69.6) | 19,207 (68.7) | 1.03 (0.98–1.08) |
∗ HR adjusted by age at the start date, gender, and low-dose aspirin indication.
† Number of concomitant medications during the month before the first aspirin prescription.
‡ Within the year before the start date.
§ As defined by consensus publications.
| Comedication | Aspirin Discontinuers, n = 7,636 (%) | Aspirin Nondiscontinuers, n = 27,968 (%) | HR ∗ (95% CI) |
|---|---|---|---|
| H 2 antagonists | |||
| Nonuse | 7,097 (92.9) | 26,066 (93.2) | 1.0 |
| Current use | 291 (3.8) | 1,078 (3.9) | 1.00 (0.89–1.13) |
| Warfarin | |||
| Nonuse | 7,354 (96.3) | 27,059 (96.7) | 1.0 |
| Current use | 199 (2.6) | 621 (2.2) | 1.30 (1.13–1.49) |
| Clopidogrel | |||
| Nonuse | 7,091 (92.9) | 24,268 (86.8) | 1.0 |
| Current use | 471 (6.2) | 3,515 (12.6) | 0.56 (0.51–0.62) |
| Dipyridamole | |||
| Nonuse | 7,487 (98.0) | 27,132 (97.0) | 1.0 |
| Current use | 123 (1.6) | 784 (2.8) | 0.61 (0.51–0.73) |
| Statins | |||
| Nonuse | 3,787 (49.6) | 11,027 (39.4) | 1.0 |
| Current use | 3,624 (47.5) | 16,425 (58.7) | 0.66 (0.63–0.70) |
| Nitrates | |||
| Nonuse | 5,278 (69.1) | 18,928 (67.7) | 1.0 |
| Current use | 1,703 (22.3) | 7,229 (25.8) | 0.80 (0.75–0.85) |
| Digoxin | |||
| Nonuse | 7,363 (96.4) | 27,158 (97.1) | 1.0 |
| Current use | 236 (3.1) | 691 (2.5) | 1.37 (1.20–1.56) |
| Antihypertensives | |||
| Nonuse | 1,976 (25.9) | 4,762 (17.0) | 1.0 |
| Current use | 5,344 (70.0) | 22,437 (80.2) | 0.60 (0.57–0.63) |
| tNSAID | |||
| Nonuse | 5,820 (76.2) | 21,170 (75.7) | 1.0 |
| Current use | 648 (8.5) | 2,577 (9.2) | 0.89 (0.82–0.96) |
| Coxibs | |||
| Nonuse | 7,220 (94.6) | 26,571 (95.0) | 1.0 |
| Current use | 167 (2.2) | 534 (1.9) | 1.06 (0.91–1.24) |
| Among women | |||
| HRT | |||
| Nonuse | 3,050 (88.4) | 11,115 (91.0) | 1.0 |
| Current use | 227 (6.6) | 665 (5.5) | 0.99 (0.86–1.13) |
∗ Adjusted by age at the start date, gender, and low-dose aspirin indication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


