A retrospective cohort analysis was conducted on patients who underwent percutaneous coronary intervention (PCI) before and after a practice change which reduced the infusion duration of eptifibatide from 18 hours to the time required for completion of a single vial of 75 mg initiated during PCI. Primary end points were inhospital cardiovascular events, target vessel revascularization, and major or minor bleeding. The secondary end point was drug cost. A total of 1,647 patients received the standard-duration infusion (18 hours), and 1,237 received the short-duration infusion. The median infusion times were 18.1 hours (interquartile range 17.7 to 18.7) and 6.6 hours (interquartile range 5.6 to 11.3) in the standard- and short-duration groups, respectively. No differences were found for the rate of inhospital cardiovascular events (2.0% vs 1.9%, respectively; p = 0.78) or inhospital revascularization (0.2% vs 0.3%, respectively; p = 0.68). Also, no statistically significant difference was observed in major bleeding (standard 4.3% vs short 4.4%; p = 0.94) or minor bleeding (standard 3.3% vs short 2.3%; p = 0.09). In conclusion, using a shortened infusion reduced eptifibatide use by an average of 1.6 vials at cost savings of $823 per patient and resulted in no difference in inhospital cardiovascular events, revascularization, or bleeding.
Highlights
- •
It is not clear whether an 18-hour eptifibatide is better than a short infusion for percutaneous coronary intervention.
- •
A practice change reduced 18-hour infusion of eptifibatide to a single vial of 75 mg.
- •
Inhospital cardiovascular events did not change with the shortened infusion.
- •
Inhospital major and minor bleeds did not change with the shortened infusion.
- •
Short infusion resulted in cost savings of $823 per patient on the basis of 2014 average wholesale price.
Trauma-related arterial thrombogenicity persists up to 18 to 24 hours after percutaneous coronary intervention (PCI), and glycoprotein IIb/IIIa inhibitors (GPI) reduce the trauma-mediated ischemic complications of PCI. However, the standard 18- to 24-hour infusion duration of the GPI eptifibatide was established before oral dual-antiplatelet therapy (DAPT) loading became standard practice. Clopidogrel loading doses of 600 and 300 mg achieve adequate antiplatelet effect within 2 and 6 hours, respectively, thereby raising the question of whether the standard 18- to 24-hour eptifibatide infusion is necessary or if a shorter infusion is sufficient. Administration of an eptifibatide bolus produces an early onset of antiplatelet effect which is maintained throughout the subsequent infusion. Recovery of platelet function does not occur until 2 to 4 hours after discontinuation of the infusion. A single 75-mg eptifibatide vial administered at recommended rates requires a minimum of 5-hour infusion (maximum rate of 15 mg/hour); therefore, the duration of antiplatelet effect provided by the infusion of a single 75-mg vial should be sufficient given the time to onset of the clopidogrel loading dose. Thus, an institution-wide practice change was made from an 18-hour eptifibatide infusion to an infusion limited to the time required to infuse one 75-mg vial when eptifibatide was used as adjunctive therapy for PCI. This study was conducted to evaluate how this change impacted inhospital cardiovascular events, bleeding, and eptifibatide cost during the index hospitalization.
Methods
A single-center, retrospective cohort analysis was conducted using the electronic medical record of 2,884 patients stratified to standard- (18 hours) or short-duration (infusion of a single 75-mg vial) eptifibatide infusion who underwent PCI from 2007 through 2011 ( Figure 1 ). From January 2007 to May 2009, all patients received the standard infusion per institutionally approved practice (standard-duration group). From June 2009 to December 2011, following an institutional practice change, eptifibatide infusions initiated during PCI were shortened (short-duration group) to the time required to infuse a single 75-mg vial (not to exceed 18 hours). Eptifibatide was the institution’s preferred GPI during both study periods. All patients aged ≥18 years who received an eptifibatide infusion as adjunctive therapy for PCI from January 2007 through December 2011 were identified (n = 3,048). One hundred sixty-four patients were excluded because they did not authorize their medical record for research. Both practices recommended eptifibatide 2 μg/kg/min (maximum 15 mg/hour) after a double bolus of 180 μg/kg (maximum 22.6 mg) 10 minutes apart. The infusion was reduced to 1 μg/kg/min (maximum 7.5 mg/hour) for patients with renal dysfunction (creatinine clearance <50 ml/min).
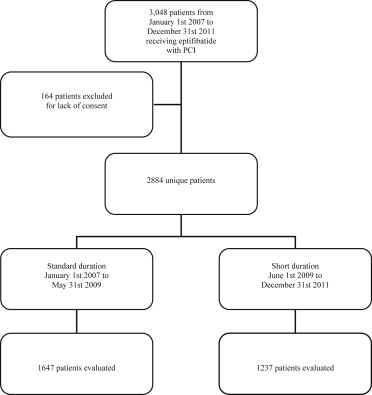
The data from patients who underwent PCI were entered into a PCI registry, and data were electronically pulled and stored in a central database. Only patients who had consented to the use of their medical information for research were included in this registry. Prespecified clinical and procedural data of inhospital events and laboratory values were obtained from patient charts. Personnel responsible for database abstraction were unaware of study objectives. Baseline and clinical data were retrospectively collected and analyzed from the database. Primary end points were cardiovascular events (death, ST elevation myocardial infarction [STEMI], coronary artery bypass graft [CABG], or ischemic stroke as a composite and each individually), target vessel revascularization (TVR), major bleeding (any central nervous system [CNS], retroperitoneal, gastrointestinal bleed, or the requirement of blood transfusion), and minor bleeding (any hematoma or vascular site bleed) for the index hospitalization. The secondary end point was cost represented as the 2014 average wholesale price (AWP). To quantify the number of vials for the standard-duration group, the median body weight was used to calculate a dose and number of vials per patient. The number of vials per patient was compared with the 1 vial per patient used by the short-duration group.
Inhospital death was recorded for death from any cause. Fatal and nonfatal STEMI were included and classified by guideline definitions at the time of PCI. The end point TVR was defined as urgent intervention of the same lesion in the same hospitalization. Recognition of new ischemic stroke, CNS, or retroperitoneal bleed was confirmed with computed tomography. Blood transfusion was defined by a minimum of 1 unit of red blood cells.
Patients who underwent PCI during the study periods received heparin, aspirin, and clopidogrel per standardized protocol. Weight-adjusted heparin was administered to maintain an activated clotting time of 200 to 300 seconds, and use of heparin was discontinued after PCI. Before PCI, patients received loading doses of aspirin (325 mg) and clopidogrel (300 to 600 mg).
Continuous variables were summarized as median and interquartile range (IQR) and discrete variables as frequencies and percentages. Baseline comparisons between the 2 eptifibatide duration groups were done using Wilcoxon rank-sum tests or Pearson chi-square tests for continuous and discrete variables, respectively. Inhospital event rates were compared between eptifibatide duration groups using the Pearson chi-square or Fisher’s exact tests. The alpha level of 0.05 was used to assess statistical significance.
In accordance with the amended Declaration of Helsinki, this study was approved by the Mayo Clinic Institutional Review Board (PR12-007351-02).
Results
Standard-duration infusions were assessed in 1,647 patients before the practice change and short infusion in 1,237 patients after the practice change. Baseline characteristics ( Table 1 ) were similar between the 2 groups; with the exception that the standard group had greater pre-PCI platelet counts per liter, fewer radial catheterizations, and more elective PCI. The median eptifibatide infusion time was 18.1 hours (IQR 17.7 to 18.7) in the standard-duration group and 6.6 hours (IQR 5.6 to 11.3) in the short-duration group. Rate of inhospital cardiovascular events and TVR ( Table 2 ) did not differ between the 2 groups. No dissimilarity in the rate of major or minor bleeds was observed ( Table 3 ). Conversion to a short-duration infusion protocol reduced eptifibatide use by 1.6 vials (75 mg/vial) per patient at a cost savings of $823 per patient on the basis of 2014 AWP for a single 75-mg vial ($514.80).
| Variable | Standard Duration Infusion (N=1647) | Short Duration Infusion (N=1237) | P Value |
|---|---|---|---|
| Age at PCI, median (IQR), (years) | 66.9 (56.9-75.7) | 67.3 (57.8-76.1) | 0.13 |
| Weight, median (IQR), (kg) | 88.0 (77.0-100.0) | 88.0 (76.0-102.0) | 0.86 |
| Men | 1177 (71%) | 903 (73%) | 0.36 |
| Pre-PCI Serum Creatinine, median (IQR),(mg/dL) | 1.0 (0.8-1.2) | 1.0 (0.8-1.1) | 0.08 |
| Creatinine clearance, median (IQR), (mL/min) | 88.2 (64.2-115.5) | 88.8 (66.3-117.6) | 0.51 |
| Hemoglobin pre-PCI, median (IQR), (g/dL) | 13.8 (12.5-14.9) | 13.9 (12.5-14.9) | 0.61 |
| Platelet pre-PCI, median (IQR), (thousand platelets/L) | 223.0 (183.0-265.0) | 210.0 (177.0-250.0) | <0.001 |
| Time from PCI to hospital discharge, median (IQR), (days) | 1 (1-3) | 2 (1-3) | 0.93 |
| Infusion duration, median (IQR), (hours) | 18.1 (17.7-18.7) | 6.6 (5.6-11.3) | <0.001 |
| History of hypertension | 1211 (78%) | 937 (77%) | 0.31 |
| Total cholesterol ≥240 | 1239 (83%) | 992 (82%) | 0.52 |
| Prior heart failure | 220 (14%) | 194 (16%) | 0.12 |
| Ejection fraction ≤ 40% | 124 (8%) | 118 (10%) | 0.05 |
| Prior Myocardial Infarct (>7 days ago) | 381 (23%) | 280 (23%) | 0.64 |
| Prior cerebral vascular attack or transient ischemic attack | 131 (8%) | 105(9%) | 0.61 |
| Prior Tumor/Lymphoma/Leukemia | 221 (13%) | 162 (13%) | 0.79 |
| Diabetes mellitus | 442 (27%) | 330 (27%) | 0.88 |
| Smoker status | 0.19 | ||
| Never | 523 (33%) | 436 (36%) | |
| Former | 707 (45%) | 504 (41%) | |
| Current | 355 (22%) | 278 (23%) | |
| Indication for PCI | |||
| Elective | 497 (30%) | 289 (23%) | <0.001 |
| Non-ST-Elevation Myocardial Infarction | 742 (45%) | 619 (50%) | 0.008 |
| ST-Segment Elevation Myocardial Infarction | 408 (25%) | 329 (27%) | 0.27 |
| Radial or femoral catheterization | |||
| Radial | 106 (6%) | 199 (18%) | <0.001 |
| Femoral | 1537 (94%) | 885 (81%) | <0.001 |
| Both | 0 | 15 (1%) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


