SECTION 1
CLINICAL CASE PRESENTATION
A 22-year-old woman who had been previously healthy, presented with acute right-sided paresthesias, expressive aphasia, and headache followed by weakness of all four extremities. The patient had an episode of tingling and numbness in the right lower extremity one month ago for which she did not seek medical care. The symptoms resolved spontaneously.
On physical examination she was hemodynamcially stable and had decreased motor strength in all four extremities. Cardiac exam revealed tachycardia without murmurs, gallops, or rubs. The initial CT scan of the head was negative; however, an MRI done later revealed acute infarcts in the territories of both middle cerebral and posterior inferior cerebellar arteries. An echocardiogram revealed depressed left ventricular systolic function (LVEF 25%) with wall motion abnormalities suggestive of Tako-tsubo cardiomyopathy, in addition to a left atrial (LA) mass attached to the fossa ovalis. Based on location, the echocardiographic appearance and the mobility of the left atrial mass, the presumptive diagnosis was an LA myxoma (Figures 9-1-1 to 9-1-5).
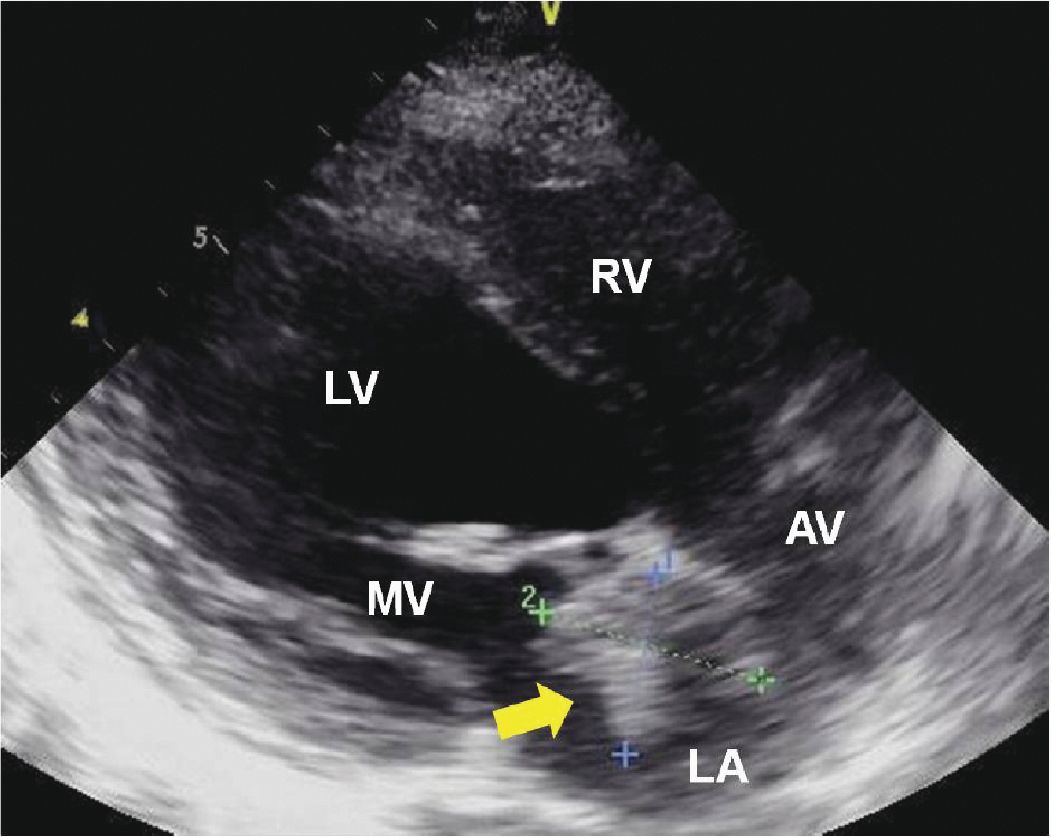
FIGURE 9-1-1 Parasternal long axis demonstrating a left atrial mass originating from the interatrial septum (arrow). RV = right ventricle, LV = left ventricle, LA = left atrium, MV = mitral valve, AV = aortic valve.
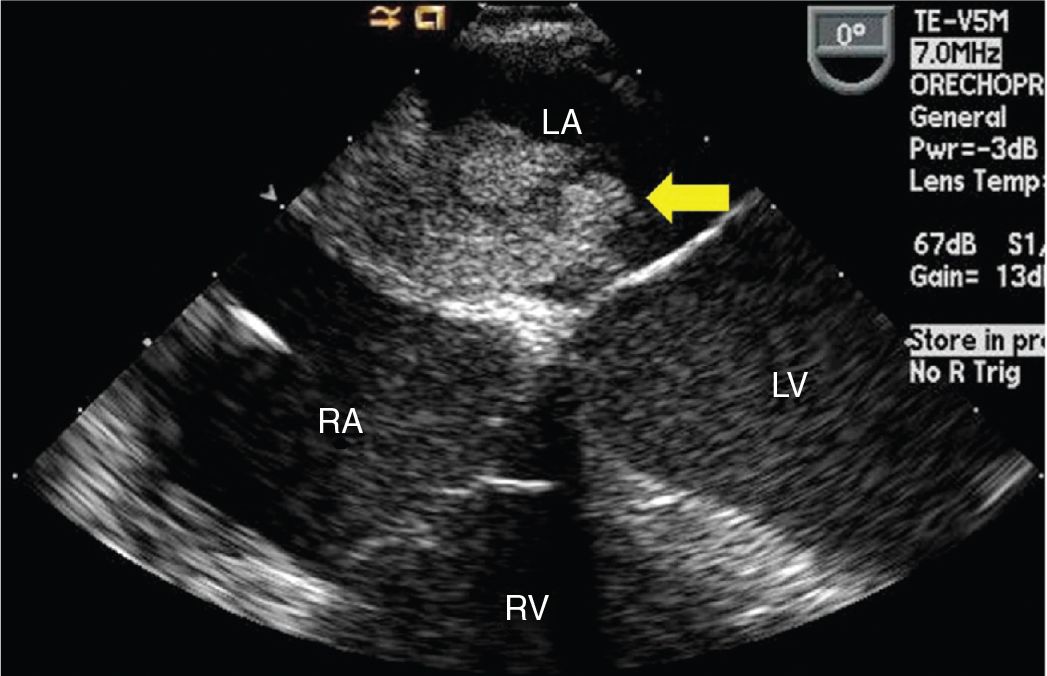
FIGURE 9-1-2 TEE midesophageal 4 chamber view showing a left atrial mass with irregular surface originating from the interatrial septum (arrow). RV = right ventricle, LV = left ventricle, RA = right atrium, LA = left atrium.
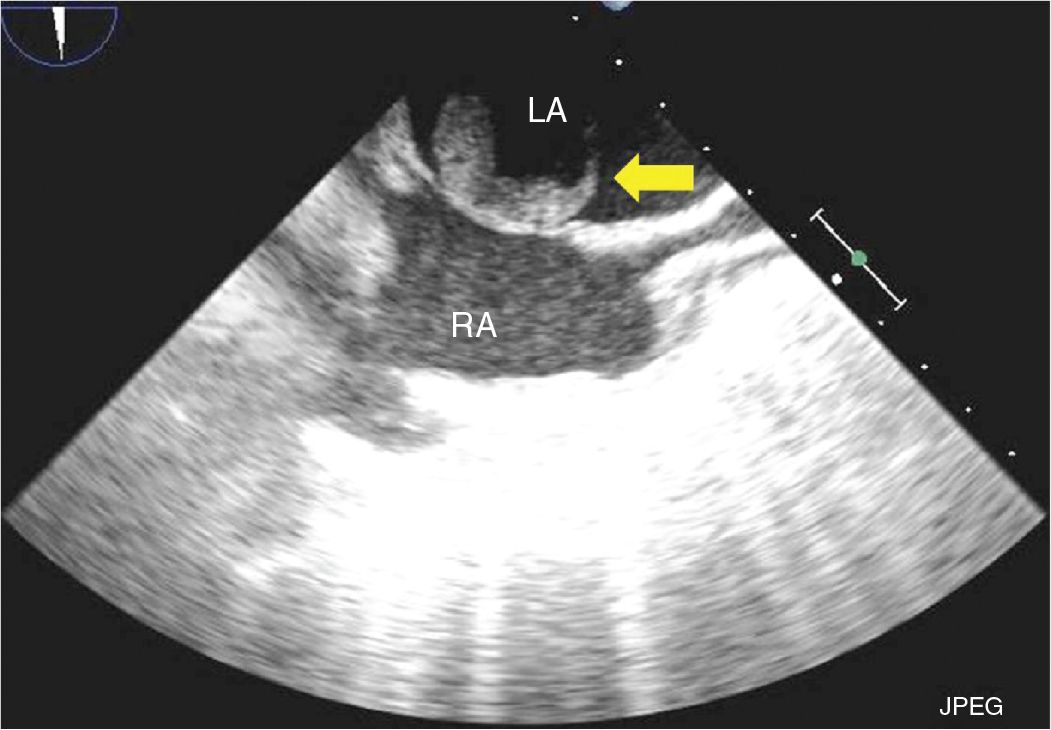
FIGURE 9-1-3 TEE bicaval view showing a left atrial mass originating from the fossa ovalis (arrow). RA = right atrium, LA = left atrium.
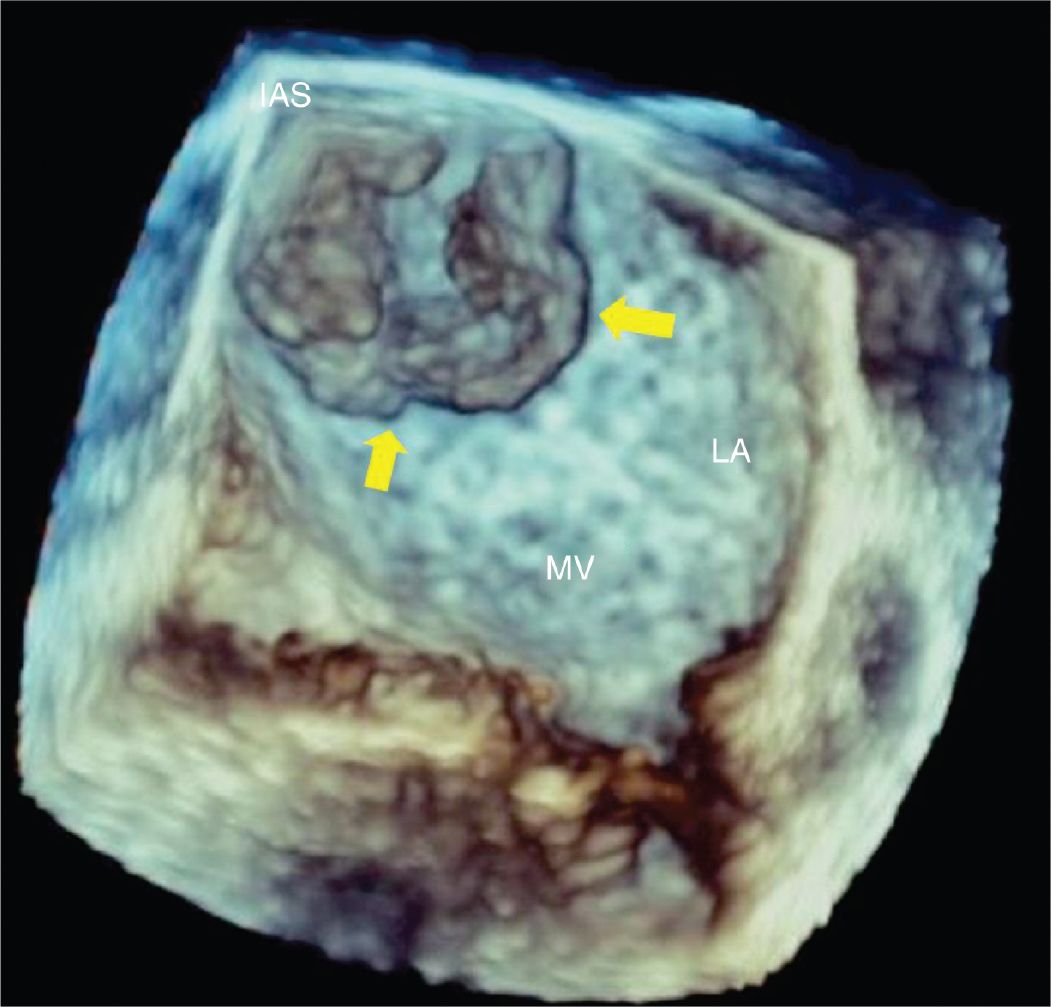
FIGURE 9-1-4 Three-dimensional TEE view of the left atrium showing a left atrial mass (arrows) originating from the interatrial septum. IAS = interatrial septum, LA = left atrium, MV = mitral valve.
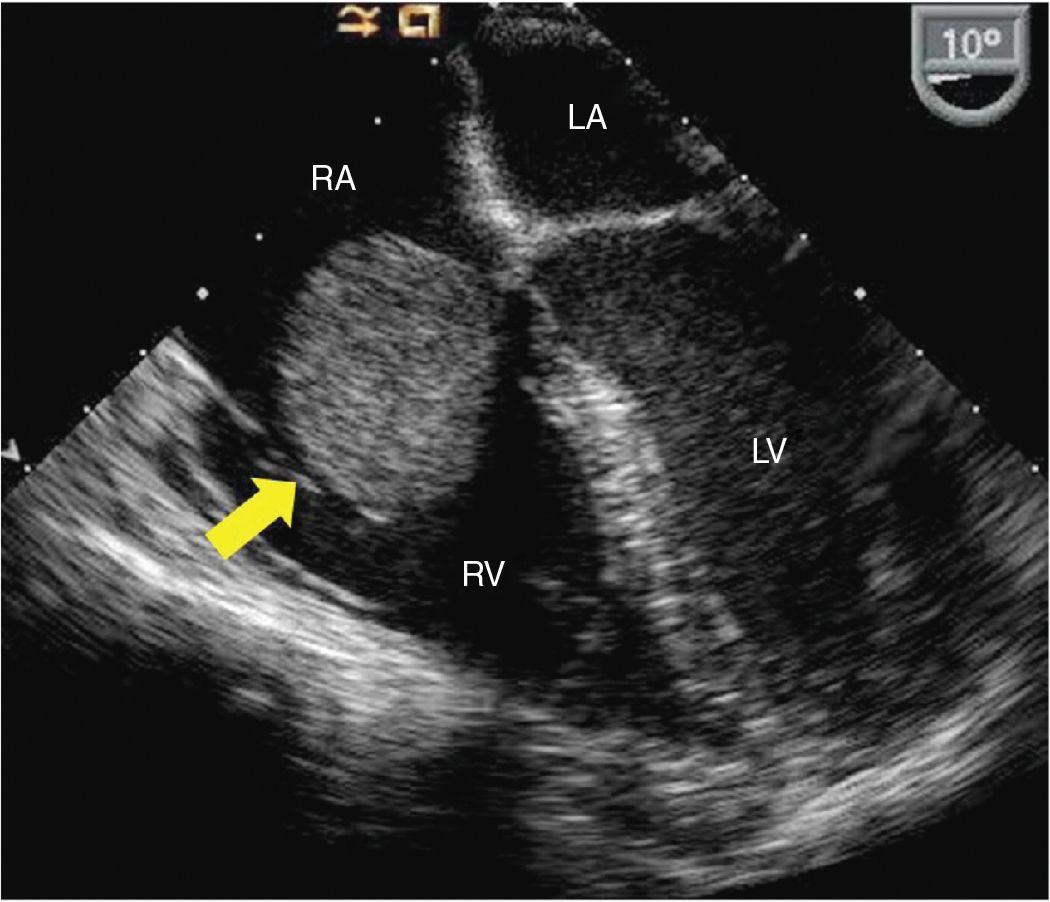
FIGURE 9-1-5 Midesophageal 4 chamber TEE view from different patient showing a right atrial mass with smooth surface, originating from the interatrial septum and prolapsing into the tricuspid valve (arrow). Pathology revealed findings consistent with a myxoma. RV = right ventricle, LV = left ventricle, RA = right atrium, LA = left atrium.
A diagnosis of multiple embolic events secondary to the left atrial mass was made, and the patient was urgently taken to the operating room for excision of the left atrial mass. The patient underwent successful excision of the left atrial mass, which was gelatinous in appearance with smooth surface in some areas and polypoid to irregular in others.
Her neurological status improved, and she regained normal strength in all four extremities except for some residual weakness in the right arm. Left ventricular systolic function improved significantly, and an echocardiogram done prior to discharge revealed LVEF of 45%.
A pathological assessment of the left atrial mass using Movat stain showed myxoid tumor with lipidic cells arranged in ring structures and cords confirming the diagnosis of left atrial myxoma.
CLINICAL FEATURES
• Patients with cardiac myxoma mostly present with one or more features of the triad of embolism, intracardiac obstruction, and constitutional symptoms. However, sometimes myxomas are incidentally detected on noninvasive cardiac imaging done for unrelated reasons. Like other cardiac tumors, the clinical features of cardiac myxoma are determined primarily by its size, location, and mobility.1
• Most common symptoms include dyspnea (45%), constitutional symptoms (27%), thromboembolism (24%), chest pain (23%), incidental (22%), palpitations (21%), atrial fibrillation (14%), syncope (12%), and ventricular tachycardia (6%).2
• Constitutional symptoms including fever, chills malaise, cachexia, and weight loss are attributable to secretion of inflammatory cytokines by the tumor or its release after tumor necrosis.
• Mechanical complications from myxoma commonly present as obstruction and/or regurgitation of the atrial ventricular valve (AV), as the tumor may prolapse through the AV valve especially when it is large and mobile. Severe obstruction may result in syncope, arrhythmia, and death.
• Symptoms related to the embolic event are primarily determined by location of the myxoma and may be the first presenting complaint in some patients. Emboli from a right sided myxoma results in pulmonary embolism. Embolism from a left sided myxoma may lead to stroke, TIA, myocardial infarction, limb and/or visceral ischemia. Systemic embolism from a right atrial myxoma in the presence of intracardiac shunt is rare.
• Myxoma can present as part of a familial syndrome known as the Carney complex, which is a multisystem tumorous disorder that features both cardiac and noncardiac myxomas (cutaneous myxoma and myxoid breast fibroadenomas), spotty skin pigmentation (lentigines and blue nevi), testicular tumors, endocrine hyperactivity (adrenocortical hyperplasia and pituitary hyperactivity), and peripheral nerve tumors (schwannomas).3 The condition is inherited as autosomal dominant trait, so screening echocardiography is recommended for all first-degree relatives. The patients with familial myxoma syndrome present at a relatively younger age; the myxomas are more likely multiple and may be found in atypical locations.1 There is a high rate of recurrence following resection. The cutaneous manifestations are a major clue to the diagnosis.
EPIDEMIOLOGY
• Primary cardiac tumors are rare with an autopsy frequency of 0.01% to 0.03%.1 Myxomas are the most common cardiac tumors, accounting for 30% to 50% of all benign cardiac tumors.
• While the majority of the myxoma cases occur sporadically, approximately 7% of cases present as a familial syndrome with an autosomal dominant pattern of transmission.
• Myxomas are most commonly diagnosed between the third and sixth decades with a mean age of approximately 50 years at presentation.4 Women are more commonly affected.
• Cardiac myxomas usually develop in the atria, with 75% originating in the left atrium and 15% to 20% originating in the right atrium.1 Myxomas may be sessile or attached to the interatrial septum with a short peduncle.
PATHOPHYSIOLOGY AND ETIOLOGY
• Cardiac myxomas are either polypoid with a smooth, gently lobulated surface or papillary with friable, irregular appearing surface. Polypoid myxomas are usually well organized, and spontaneous fragmentation is rare, while papillary myxomas pose a higher risk of embolic complications. Myxomas internally may have areas of necrosis, cyst formation, and, rarely, calcification.1
• Characteristic histological finding of myxomas include the presence of lipidic cells in a stroma rich in glycosaminoglycans.
• Size may range from 1 to 15 cm; typical diameter at presentation is 4 to 8 cm.
ECHOCARDIOGRAPHY
• Echocardiography is the diagnostic modality of choice for detection of cardiac myxoma as it can not only accurately define the location and morphology, but also provide key information about mobility and hemodynamic effects. Transthoracic echocardiography is usually sufficient to make a diagnosis; however, transesophageal echocardiogram may also be employed in cases with suboptimal images.
• Cardiac myxomas typically appear as a mobile mass attached to the endocardial surface, usually the interatrial septum by a short peduncle.
• Myxomas usually have heterogeneous echogenicity due to areas of necrosis, cyst formation, and, rarely, calcification within primary tumor.
• Doppler echocardiography is used to demonstrate hemodynamic effects of myxoma, including valvular stenosis and/or regurgitation.
• Although not used routinely, contrast echocardiography can be used to demonstrate the vascularity of the cardiac mass and differentiate a vascular tumor from thrombus.
OTHER DIAGNOSTIC TESTING AND PROCEDURES
• Cardiac CT and MRI are complimentary techniques that can be used to obtain additional diagnostic information regarding tissue characterization of the cardiac mass and assessment of the extracardiac structures. A myxoma typically has heterogeneous contrast enhancement variably reflecting areas of necrosis. Areas of calcification can be best demonstrated by CT. Myxomas notably have increased signal intensity on T2 weighted images and has differential enhancement on perfusion sequences.
• Basic laboratory findings include anemia, leukocytosis, and elevated erythrocytic sedimentation rate.
DIFFERENTIAL DIAGNOSIS
• The differential diagnoses of cardiac masses is quite broad, as listed in Table 9-1-1.
• Metastatic disease of the heart is far more common than primary cardiac tumors and is discussed in detail in a later section of this chapter. The differential diagnosis of primary cardiac tumors can be categorized based on its potential of invasion and is summarized in Table 9-1-2.
TABLE 9-1-1 Differential Diagnosis of Cardiac Mass
• Thrombus
• Vegetation
• Degenerative changes: Lambl’s excrescences
• Calcification: caseous necrosis of mitral annulus
• Tumor: benign, malignant, and metastatic
• Normal structure/variant: prominent Chiari network, Eustachian valve, crista terminalis, lipomatous hypertrophy of interatrial septum
• Iatrogenic material
TABLE 9-1-2 Differential Diagnosis of the Primary Cardiac Tumors
Benign
• Myxoma
• Papillary fibroelastoma
• Rhabdomyoma
• Fibroma
• Lipoma
• Teratoma
• Hemangioma
• Lymphangioma
• Hemangiopericytoma
• Mesothelioma of AV node
• Pericardial cyst
Malignant
• Angiosarcoma
• Rhabdomyosarcoma
• Synovial sarcoma
• Liposarcoma
• Myxosarcoma
• Fibrosarcoma
DIAGNOSIS
• Noninvasive imaging, primarily echocardiography, is the mainstay of preoperative diagnosis. Final diagnosis is often made based on appearance and pathological analysis after surgical resection of the tumor.
• Symptoms and signs of cardiac myxoma are nonspecific. Cutaneous manifestations may help in the diagnosis of familial myxoma syndrome.
MANAGEMENT
• Surgical en bloc resection of the myxoma with a margin of normal cardiac tissue is the treatment of choice and is curative in most cases. In addition to tumor resection, sometimes additional reconstruction may be warranted, eg, rare cases of myxoma arising from the mitral valve may further require valvular repair or replacement after resection of the tumor.
• After diagnosis of cardiac myxoma, surgical resection should be performed urgently to prevent embolic and/or mechanical complications like obstruction of the atrioventricular valves.5
• Surgical resection of cardiac myxomas carries low operative risk and affords excellent short and long term survival.5 A single center study from the Mayo Clinic reported that survival after successful resection of myxoma is not significantly different from age- and gender-matched population.2
FOLLOW-UP
• There is an overall 13% risk of recurrence after surgical resection, which is much more frequent in patients with familial myxomas versus isolated, sporadic tumors (22% versus 3%).1 The site of recurrence is most commonly at the location of the original tumor.2
• The cumulative incidence of recurrence increases steadily for 4 years following surgical resection, after which risk of recurrence is low. Semiannual follow-up with echocardiography has been recommended for the first 4 years following surgical resection to assess for recurrence.1 Thereafter, annual follow-up with noninvasive cardiac imaging should be considered.
REFERENCES
1. Bruce CJ. Cardiac tumours: diagnosis and management. Heart. 2011;97(2):151-160.
2. Elbardissi AW, Dearani JA, Daly RC, et al. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation. 2008;118(14 Suppl):S7-15.
3. Carney JA, Gordon H, Carpenter PC, et al. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore). 1985;64(4):270-283.
4. Burke AJ, Jeudy J Jr, Virmani R. Cardiac tumours: an update: Cardiac tumours. Heart. 2008;94(1):117-123.
5. Kuroczy´nski W, Peivandi AA, Ewald P, Pruefer D, Heinemann M, Vahl CF. Cardiac myxomas: short- and long-term follow-up. Cardiol J. 2009;16(5):447-454.
CLINICAL CASE PRESENTATION
This patient is a 38-year-old woman who presented to the emergency department after she woke up with left arm numbness, which progressed rapidly to involve her left leg. She also has a history of hypertension and migraines. The patient denied any weakness or sensory symptoms anywhere else in the body.
On physical examination she was found to have diminished proprioception, two-point discrimination on the left upper and lower extremities. She had normal muscle strength in all four extremities. Cardiovascular examination was normal. A CT scan of the head revealed a small hypo density in the right temporal lobe and external capsule region suggesting acute infarction. MRI of the brain confirmed a right middle cerebral artery territory perisylvian acute nonhemorrhagic infarction. An echocardiogram, done to assess for intracardiac source of emboli, revealed an intracardiac mass, measuring 0.8 × 0.9 cm, attached to the ventricular side of the anterior mitral leaflet, suspicious for a cardiac papillary fibroelastoma (CPF) (Figures 9-2-1 to 9-2-4).
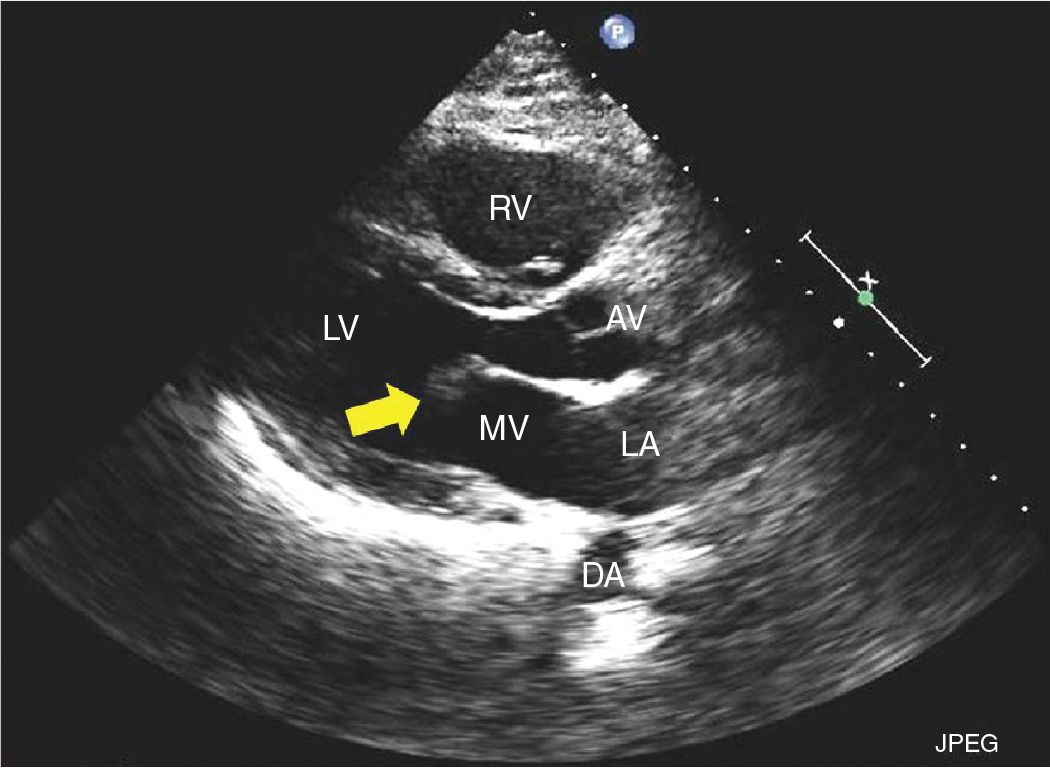
FIGURE 9-2-1 Parasternal long axis demonstrating a mass attached to ventricular side of the anterior mitral leaflet (arrow) consistent with a CPF. RV = right ventricle, LV = left ventricle, LA = left atrium, MV = mitral valve, AV = aortic valve, DA = descending aorta.
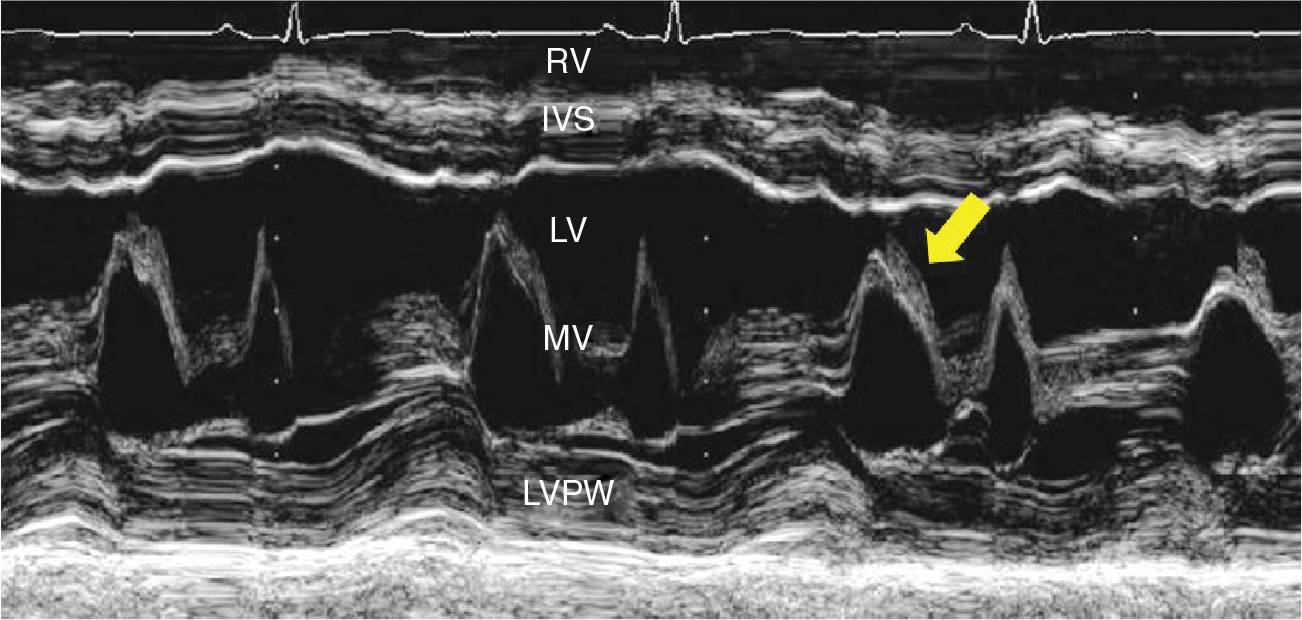
FIGURE 9-2-2 M-Mode imaging at the tip of mitral valve demonstrating the evidence of intracardiac mass (arrow).
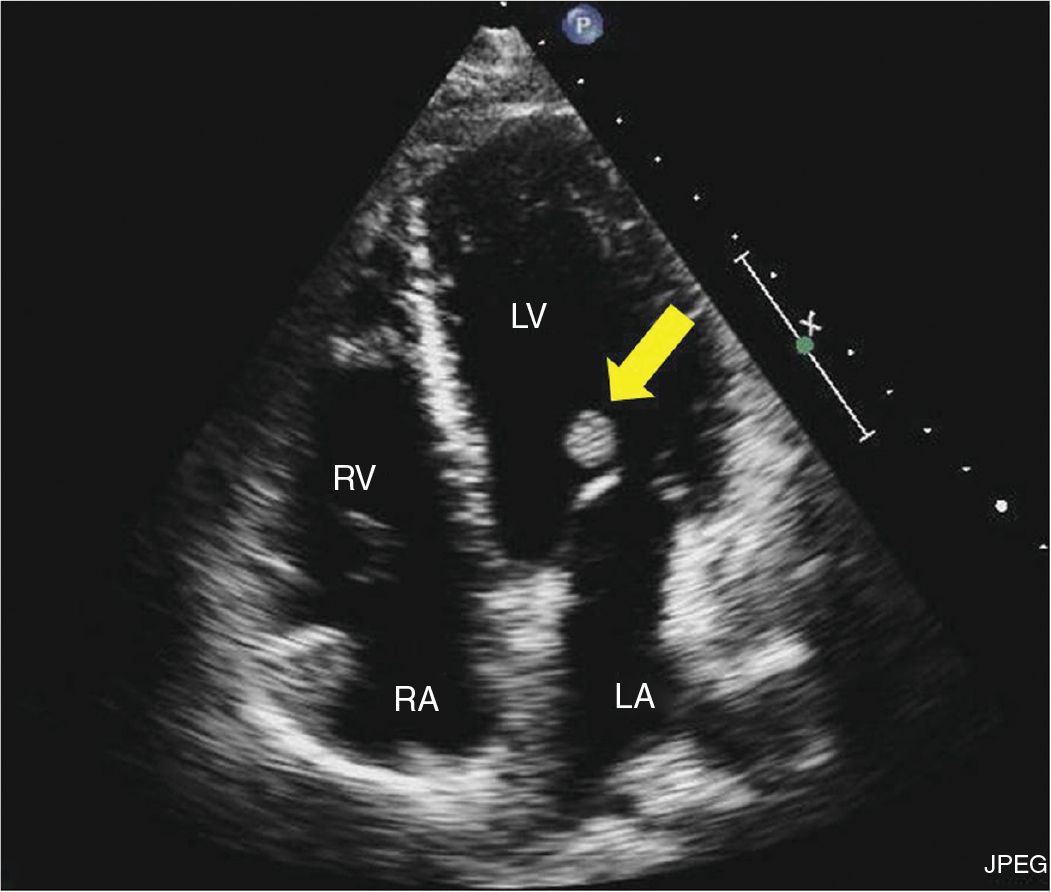
FIGURE 9-2-3 Apical 4 chamber view showing a mass attached to the ventricular side of the anterior mitral leaflet (arrow). RV = right ventricle, LV = left ventricle, LA = left atrium, RA = right atrium.
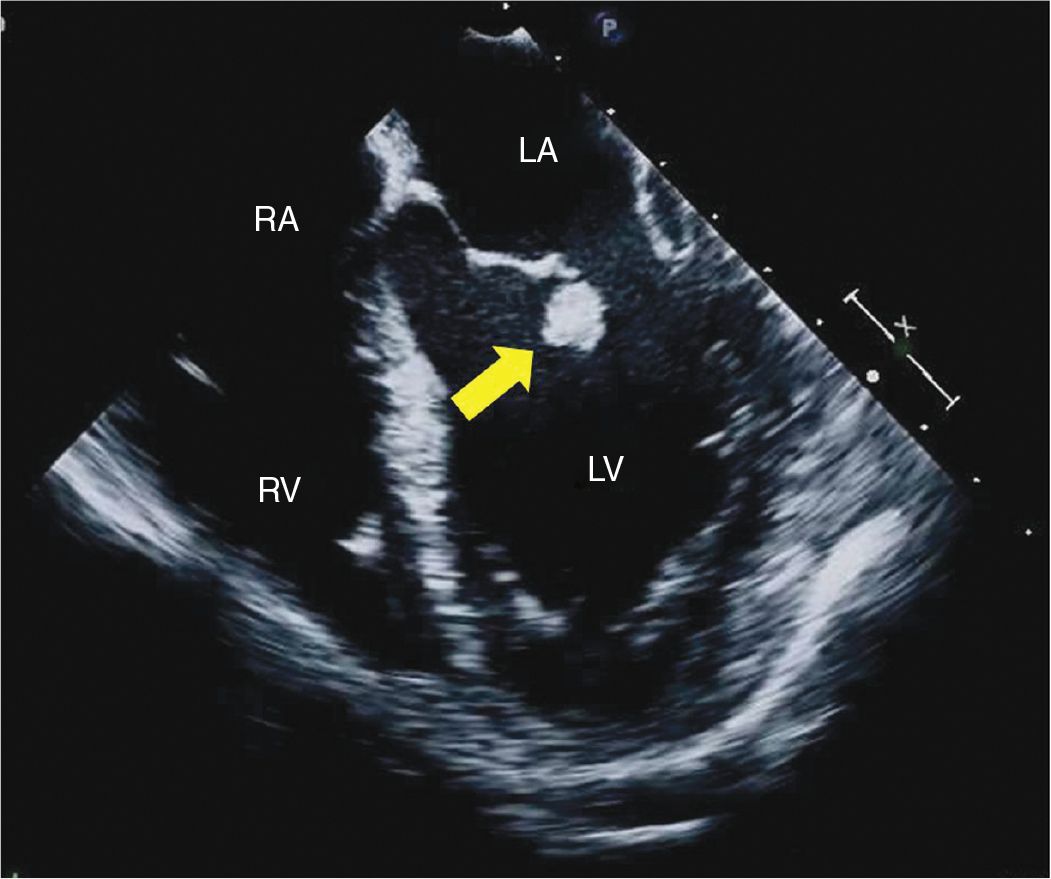
FIGURE 9-2-4 TEE midesophageal 4 chamber view demonstrating a mass attached to the ventricular side of the anterior mitral leaflet (arrow). RV = right ventricle, LV = left ventricle, LA = left atrium, RA = right atrium.
She was referred for surgical resection of the intracardiac mass. During cardiac surgery, a small mobile mass attached to the A2 segment of the anterior mitral leaflet with a short peduncle was seen and successfully excised. The patient had an uneventful postoperative course and has had no recurrence so far after 3 years of follow-up.
Surgical pathology using Movat’s pentachrome staining showed a papillary tumor with fibrous cords containing elastic fibers, which confirmed the preoperative diagnosis of cardiac papillary fibroelastoma.
CLINICAL FEATURES
• Nearly 50% of cases are diagnosed incidentally on echocardiogram done for unrelated reasons or during assessment of a cardiac source of embolism.1
• Although frequently CPF is diagnosed incidentally, transient ischemic attack, stroke, myocardial infarction, sudden death, heart failure, presyncope, syncope, pulmonary embolism, blindness, and peripheral embolism related to CPF have been reported.2
• Tumor mobility is an independent predictor of death and nonfatal embolic event.2
EPIDEMIOLOGY
• CPF is the second most common primary cardiac tumor after myxoma. In a single center study based on postoperative surgical pathology, CPF accounted for 26% of benign cardiac tumors.3 CPF accounts for approximately 75% of all cardiac valvular tumors.4
• The true prevalence of CPF is unknown, and reported data is likely an underestimation as a significant proportion of the patients remain asymptomatic. The frequency of detection of CPF has increased with improvements in echocardiographic resolution with advent of higher frequency transducers and more frequent use of TEE.5
• CPF has a wide range of age at presentation with a mean of 60 years. The highest prevalence is noted in the eighth decade followed by the fourth decade. Males comprise approximately 55% of cases.2,5
• There is a strong association between CPF and hypertrophic cardiomyopathy, cardiac surgery, hemodynamic trauma, and radiation exposure.1
PATHOPHYSIOLOGY AND ETIOLOGY
• CPF are mostly solitary, small (average 1.1 ± 0.5 cm), and originate from the valvular surface (80% versus 20% from atrial and ventricular surfaces).5
• CPF have a characteristic frond-like appearance that resembles a sea anemone on gross examination, especially when placed under saline. Histologically, the tumor is comprised of an inner avascular core of connective tissue containing collagen, smooth muscle, and elastic fibers that is surrounded by a layer of mucopolysaccharide, followed by an outermost layer on endothelium.
• CPF are considered to be slow-growing tumors, though the natural history has not been characterized.
• Embolic complications may occur due to embolization of attached thrombi or the tumor itself. CPF has been reported to prolaspe into the coronary ostium, which may lead to myocardial infarction.
ECHOCARDIOGRAPHY
• Due to superior spatial and temporal resolution, echocardiography is the principal imaging modality to diagnose and follow up CPF. TEE is an important diagnostic tool to define the extent and anatomic attachments of CPF.5
• CPF is traditionally described as a small and well-delineated mass with a predilection for valvular endocardium. Nearly 45% of CPF have a small stalk and are mobile. CPF has a characteristic echocardiograph appearance of a central homogeneous speckled pattern and a characteristic stippling along the edges with a “shimmer” or “vibration” at the tumor-blood interface.1,4,5
• CPF typically originates from the left sided cardiac valves, most commonly from the aortic valve, followed by mitral valve. CPF do not cause valvular dysfunction. The most common nonvalvular site of origin is the left ventricle.
• For CPF size more than 0.2 cm, the sensitivity, specificity, and accuracy of the transthoracic echocardiography is 88.9%, 87.8%, and 88.4%, respectively.5
• Figures 9-2-5 to 9-2-9 provide other examples of CPF.
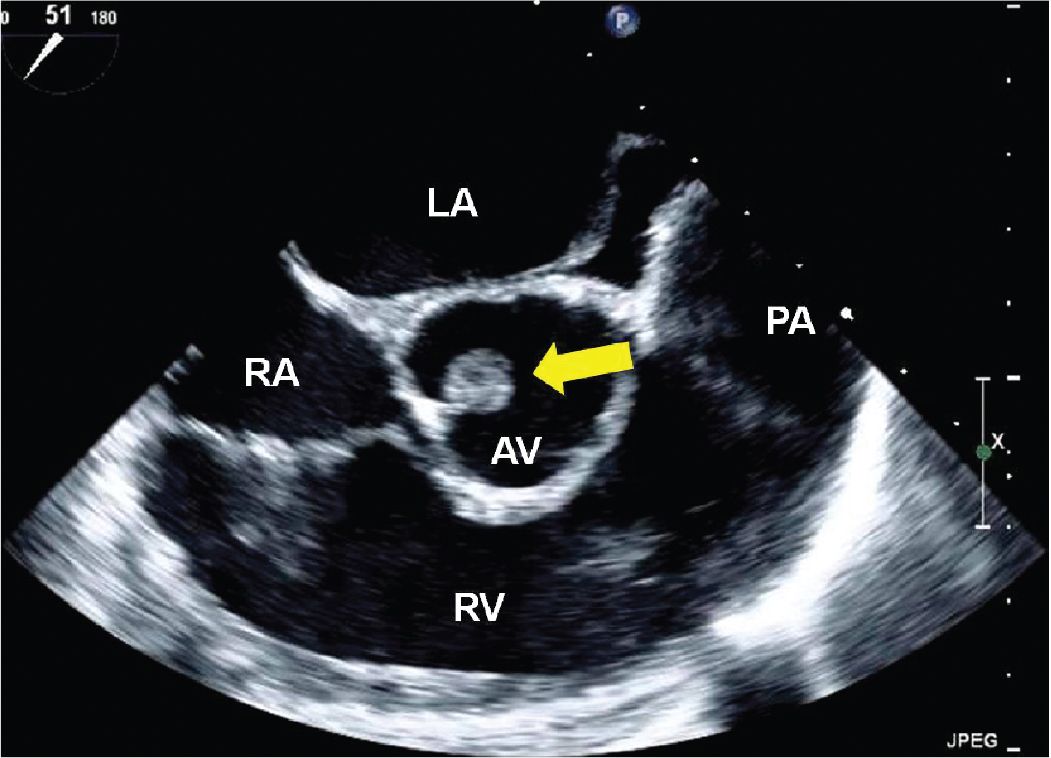
FIGURE 9-2-5 TEE midesophageal short axis aortic valve view demonstrating a mass attached to the noncoronary cusp of the aortic valve in a different patient with a CPF (arrow). RV = right ventricle, LA = left atrium, RA = right atrium, PA = pulmonary artery.
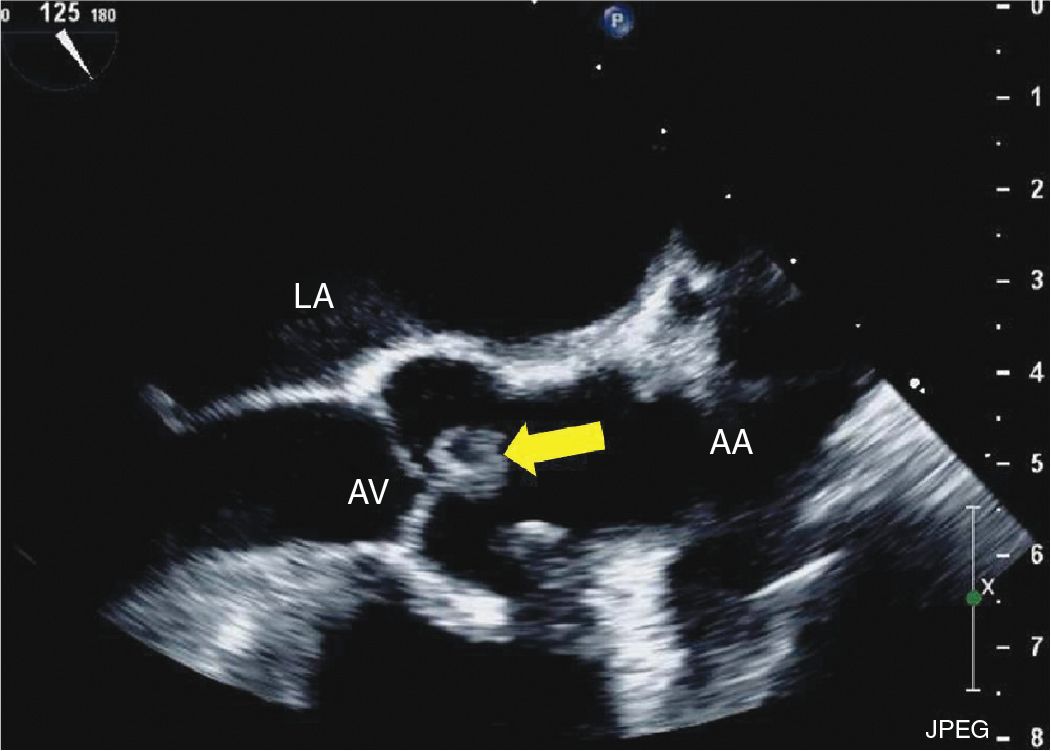
FIGURE 9-2-6 TEE midesophageal 3 chamber long axis view demonstrating a mass attached to the aortic valve in a different patient with CPF (arrow). AV = aortic valve, LA = left atrium, AA = ascending aorta.
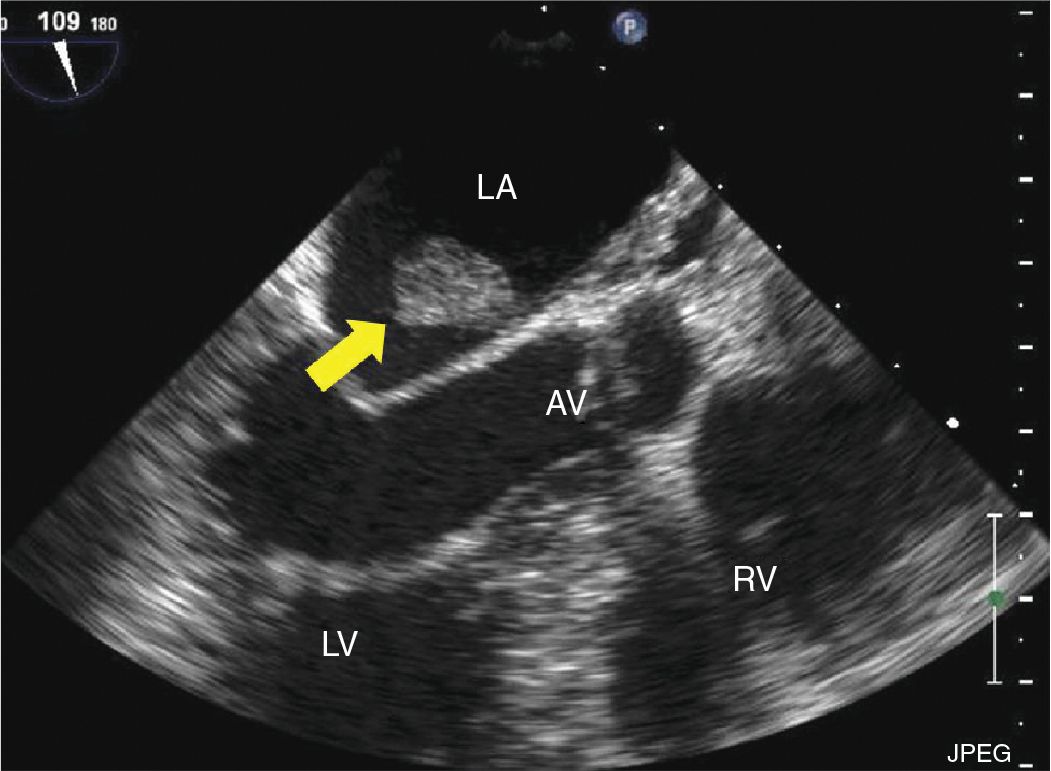
FIGURE 9-2-7 TEE midesophageal 3 chamber long axis view demonstrating a mass attached to interatrial septum in a different patient with CPF (arrow) confirmed with pathology. AV = aortic valve, LA = left atrium, RV = right ventricle, LV = left ventricle.
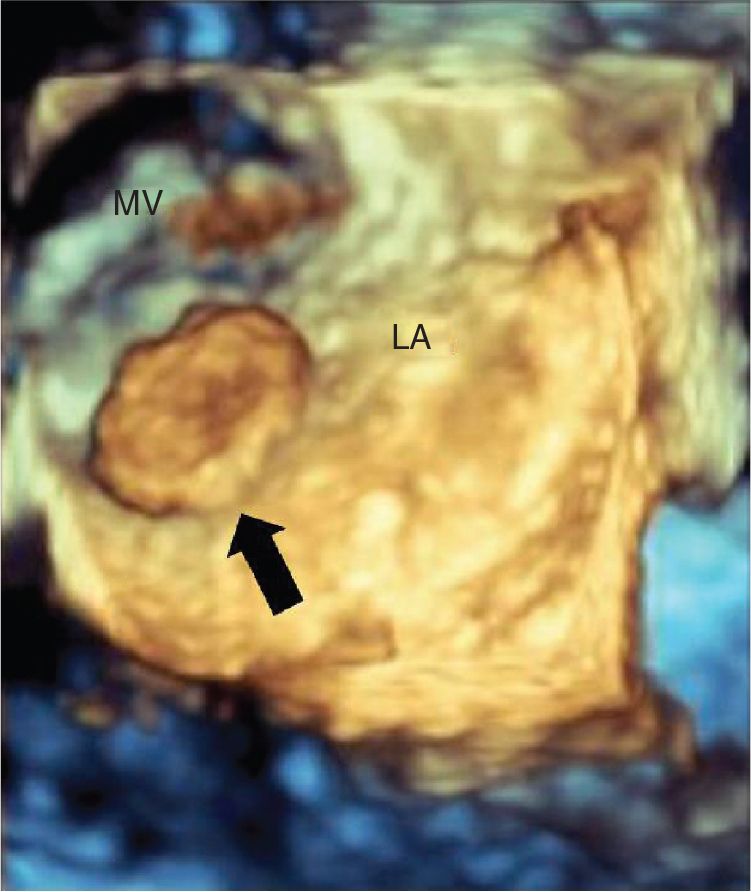
FIGURE 9-2-8 Three-dimensional view of the left atrium showing a left atrial mass originating from the interatrial septum in a different patient with CPF (arrow). LA = left atrium, MV = mitral valve.
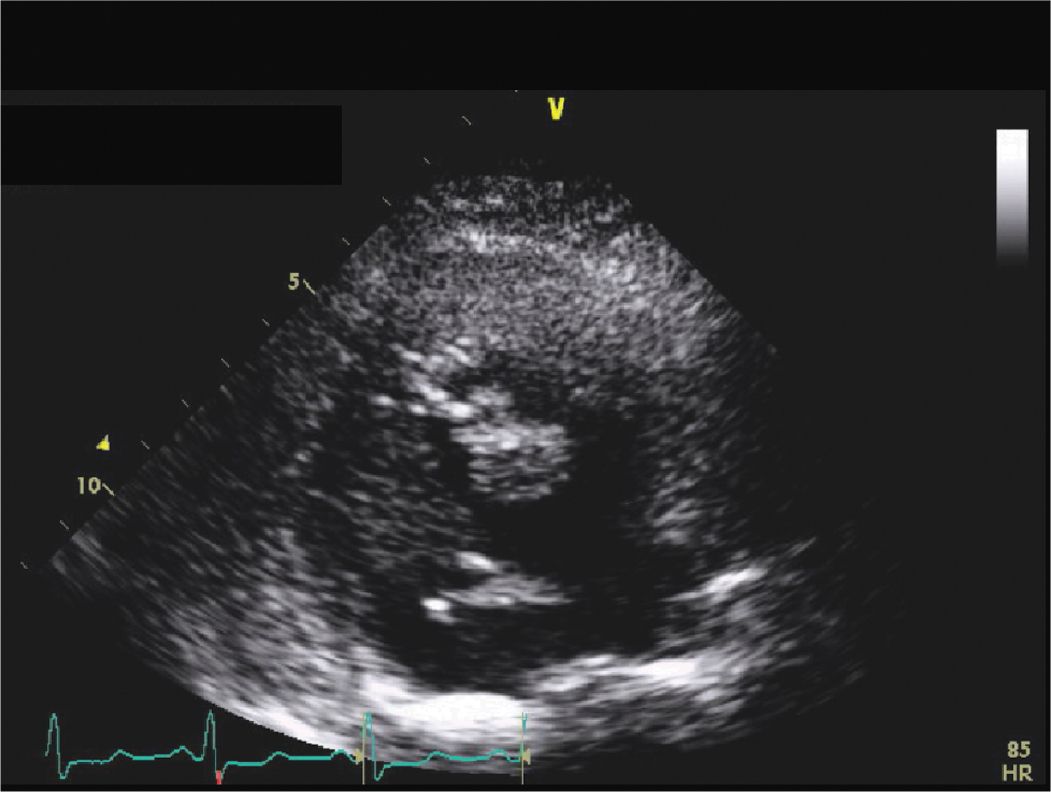
FIGURE 9-2-9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


