SECTION 1
CLINICAL CASE PRESENTATION
A 62-year-old man presented with lower extremity edema, shortness of breath, and atrial fibrillation. He had been seen in the past for coronary artery disease that was manifest by severe ischemic cardiomyopathy following an anterior myocardial infarction (MI) and a history of atrial fibrillation (AF). He had undergone coronary artery bypass surgery, mitral valve repair, pulmonary vein isolation (PVI), and stapling of left atrial appendage (LAA). His preoperative ejection fraction (EF) was 15%. Postoperatively, AF redeveloped, and he underwent radiofrequency ablation therapy (RFA) for AF. This procedure was complicated by high-grade atrioventricular (AV) block, and the following day, he underwent placement of a biventricular-pacing ICD. His EF substantially improved to 35%. He then developed gastrointestinal bleeding, which necessitated discontinuation of anticoagulation. Physical exam was unremarkable except for edema and an irregular rhythm. Prior to a planned cardioversion (CV) and in order to facilitate CV, the patient underwent TEE to assess for LA thrombus. Thrombus was identified, and the cardioversion was postponed (Figures 10-1-1 to 10-1-6). After 3 months of anticoagulant therapy, TEE was repeated and the thrombus resolved (Figure 10-1-7). He subsequently underwent CV and has done well.
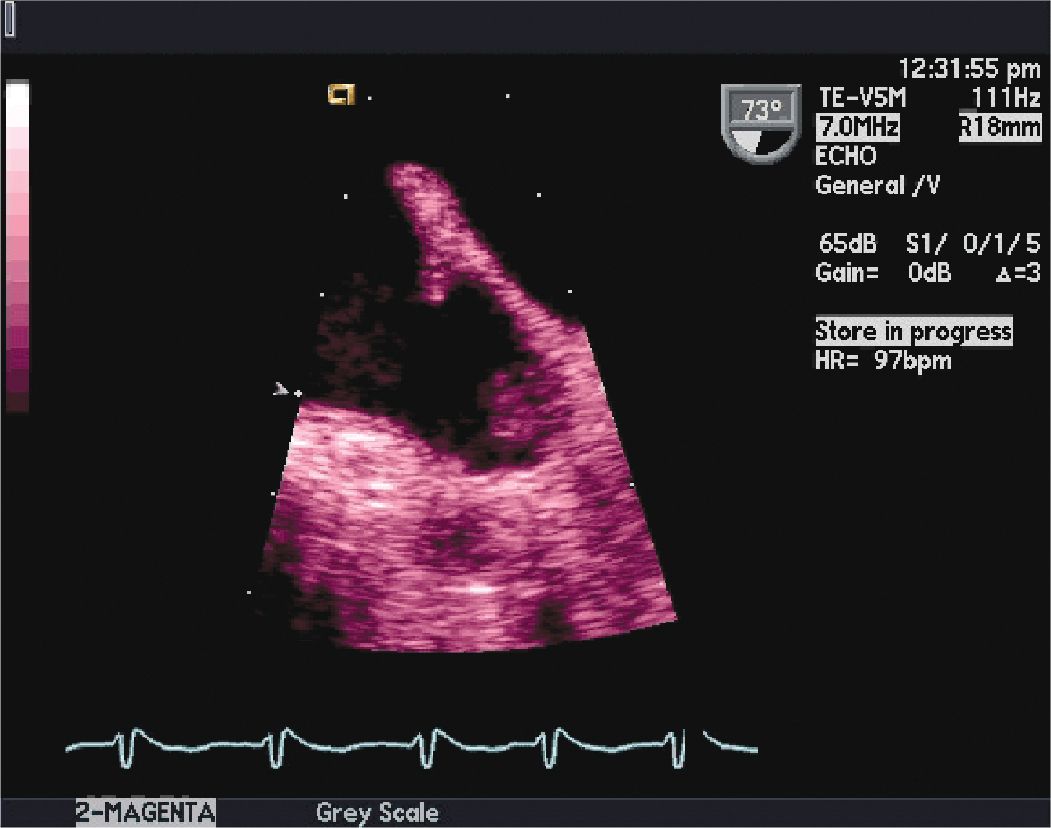
FIGURE 10-1-1 Two-dimensional TEE image at 73° in the mid-esophagus, showing the left atrial appendage LAA. This image shows thrombus attached near the apex of the LAA. The thrombus size was measured at 1.3 × 0.7 cm. The LAA is best visualized from midesophageal position. It is a complex anatomic structure and is multilobed in up to 80% of the general population. The presence of left atrial (LA) or LAA thrombus may preclude CV. The presence of LA and LAA thrombus is associated with a significantly increased risk of stroke. Following 3 weeks of therapeutic anticoagulation prior to, and at least 4 weeks of therapeutic anticoagulation after CV is recommended in the guidelines on the management of atrial fibrillation.
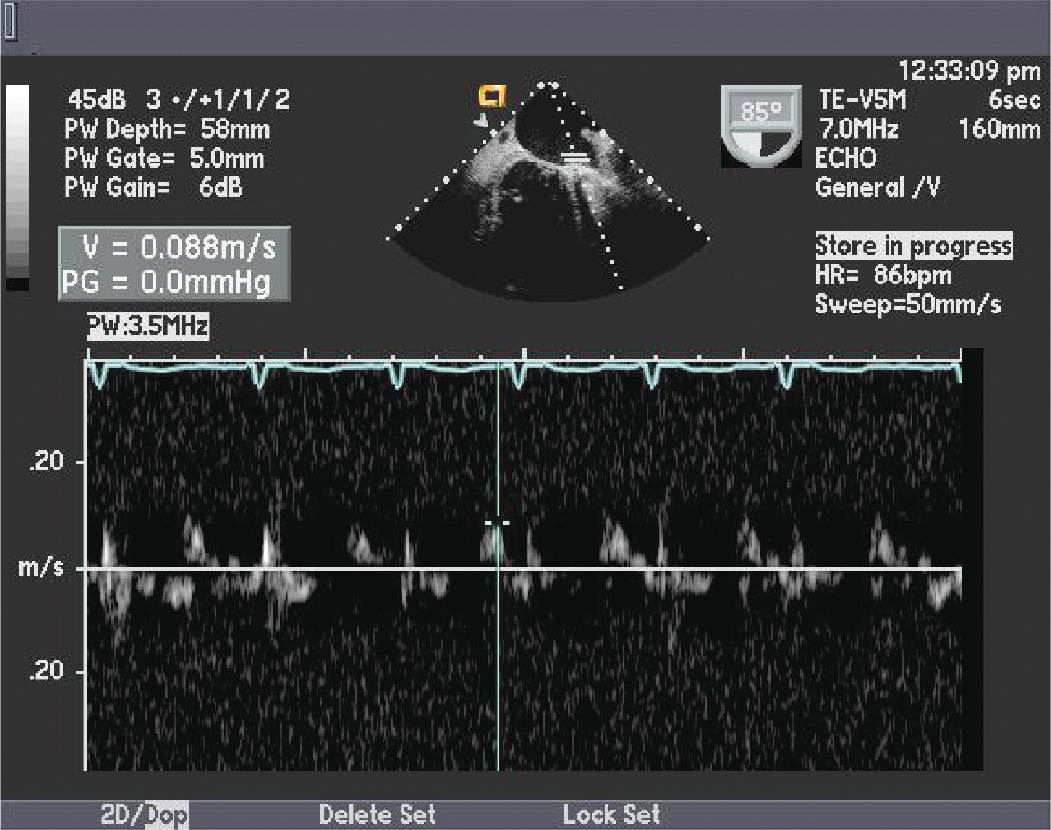
FIGURE 10-1-2 The left atrial appendage pulse wave Doppler pattern showing markedly diminished peak LAA emptying velocity. Multiple studies have found an association between low peak LAA emptying velocity (<20 cm/s) and incidence of stroke in patients with AF.
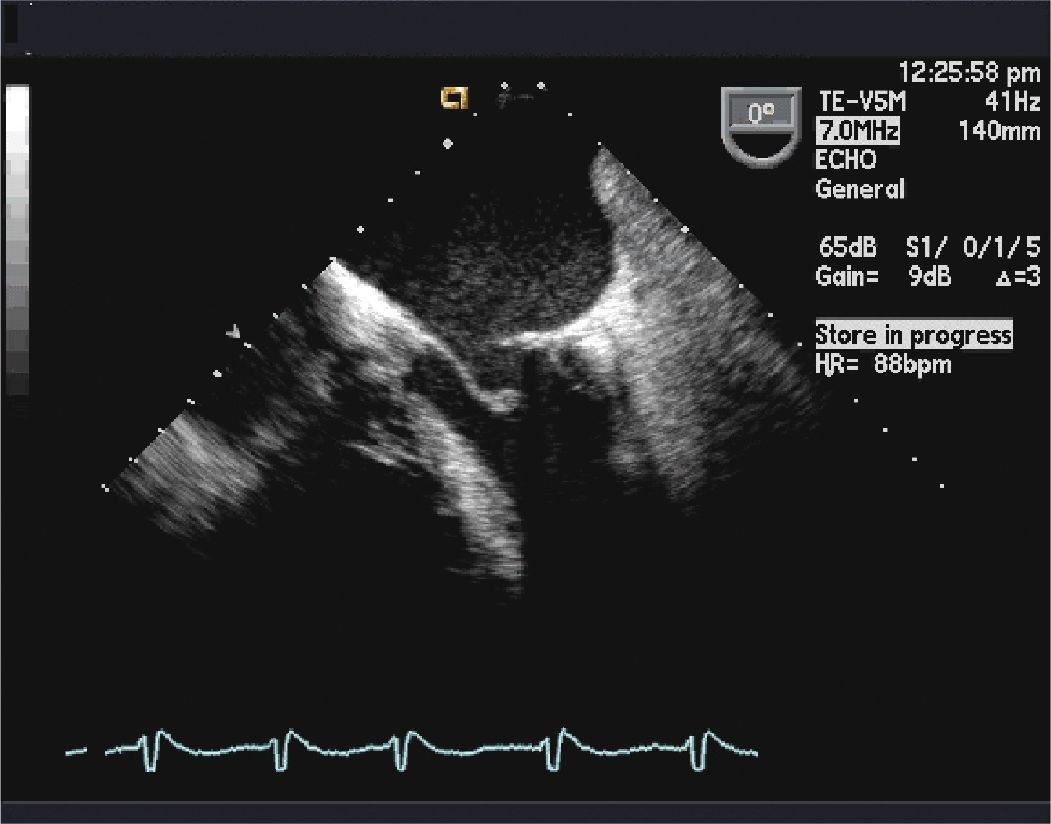
FIGURE 10-1-3 Two-dimensional TEE image at 0° in the mid-esophageous, showing the left atrium, the left ventricle, and the mitral valve. This image shows mitral ring and valve function.
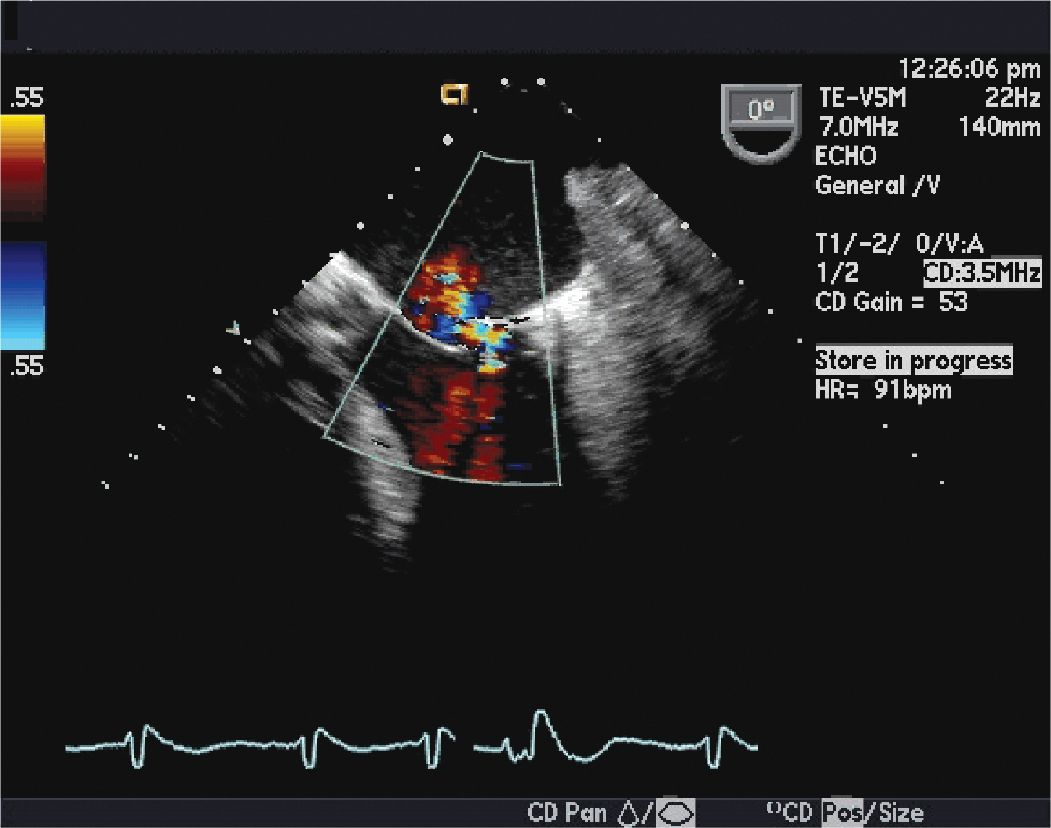
FIGURE 10-1-4 This image shows mild (1+) mitral regurgitation on the color Doppler echocardiography.
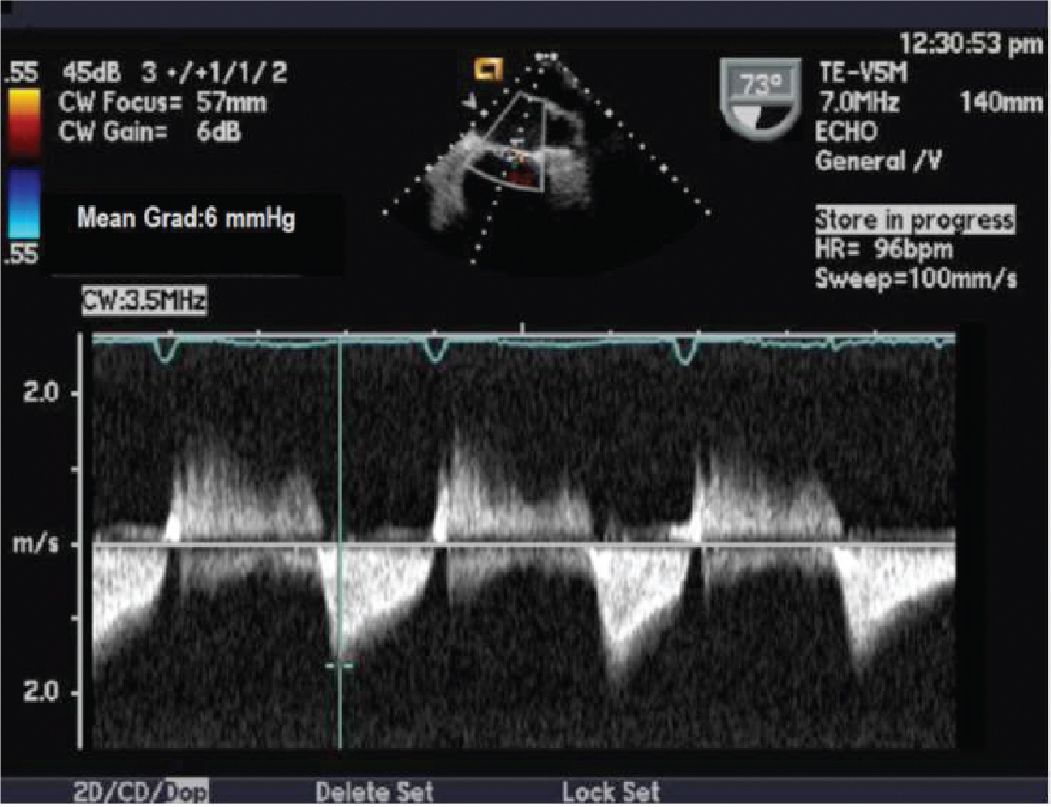
FIGURE 10-1-5 Continuous wave Doppler demonstrating mitral valve gradient (mean gradient 6 mm Hg).
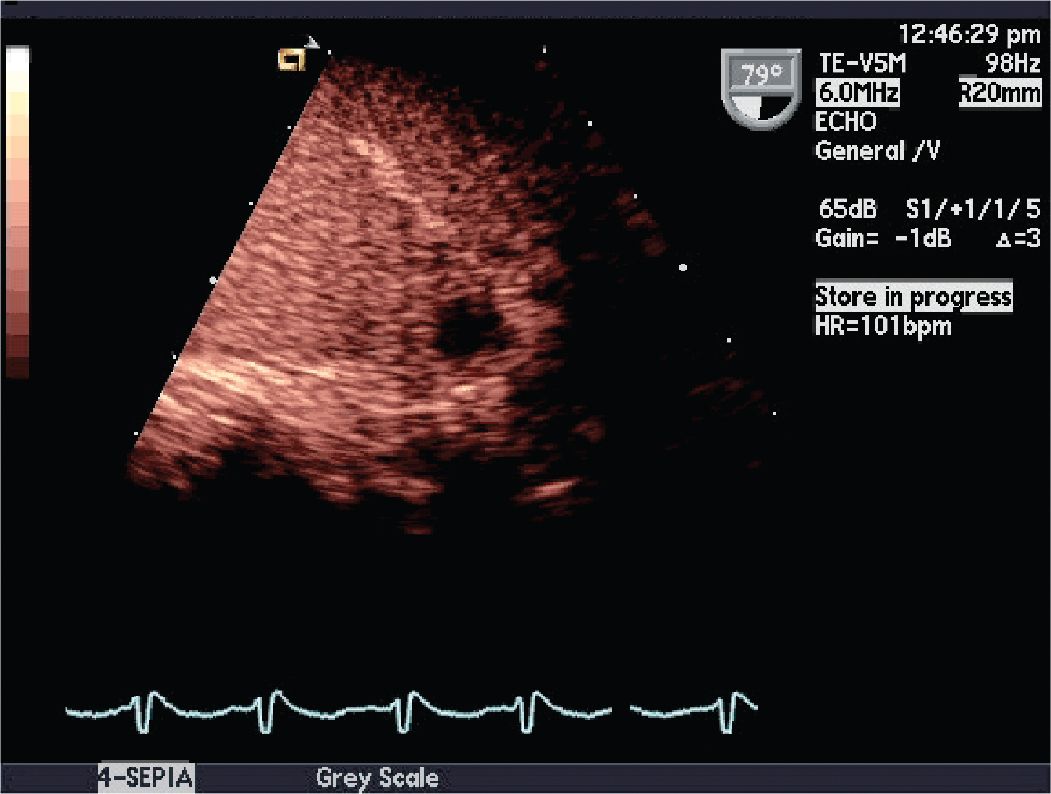
FIGURE 10-1-6 Postcontrast agent administration image demonstrating a filling defect representing an LAA thrombus.
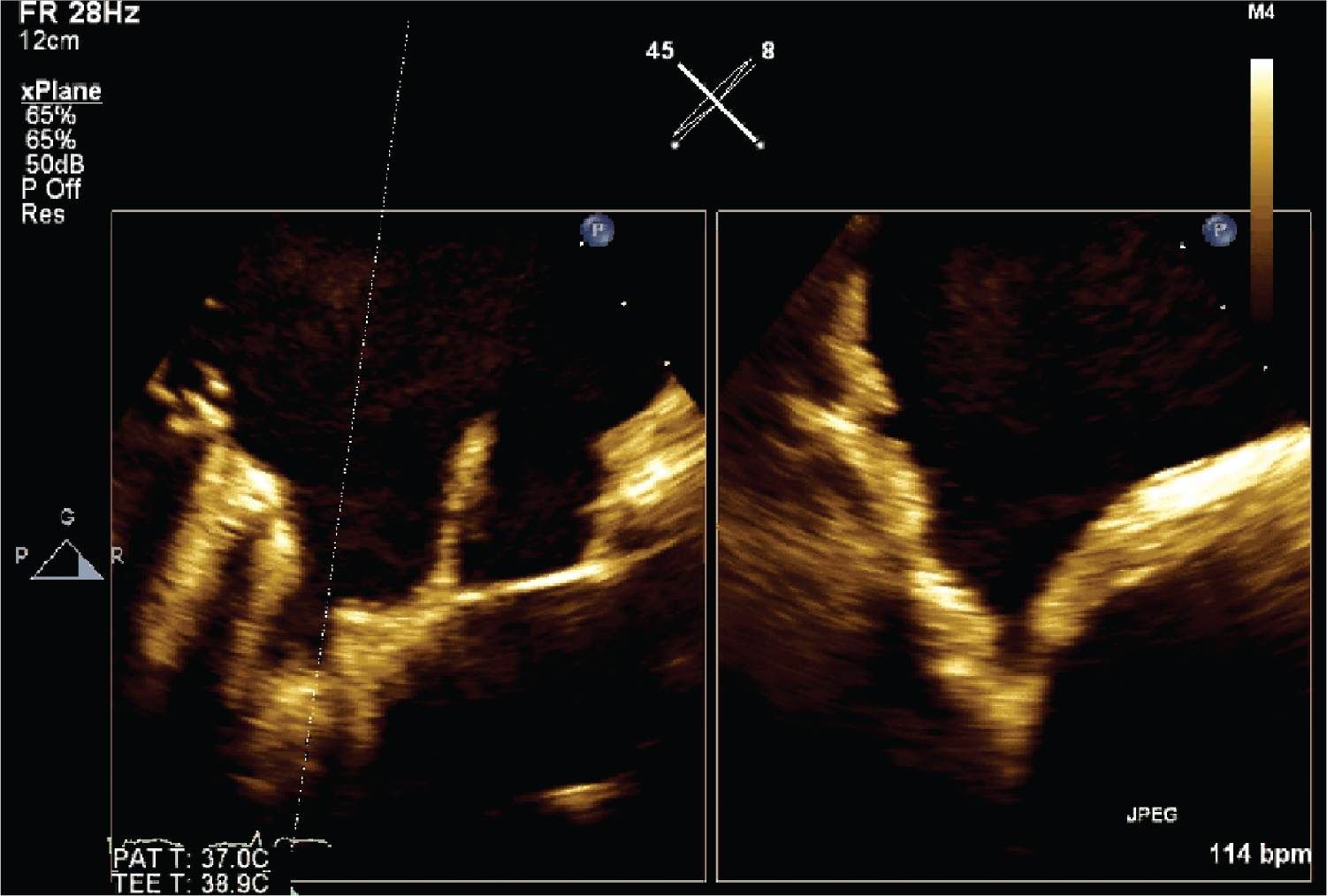
FIGURE 10-1-7 This image shows disappearance of thrombus following treatment. X-plane used to more comprehensively view orthogonal planes and hence more confidently demonstrate absence of thrombus.
CLINICAL FEATURES
• Patients may or may not have symptoms with AF.
• Commonly associated symptoms include palpitations, shortness of breath, fatigue, lightheadedness, decreasing exercise tolerance, or chest discomfort.
• An irregular pulse should raise suspicion for AF.
• Patients may present initially with transient ischemic attack (TIA) or ischemic stroke.
• Most patients experience asymptomatic episodes of arrhythmias before being diagnosed.
• Patients with mitral valve disease and heart failure often have a higher incidence of AF.
• Intermittent episodes of AF may progress in duration and frequency, and over time many patients will develop sustained AF.
• For a newly diagnosed patient with AF, reversible causes such as pulmonary embolism, hyperthyroidism, pericarditis, and MI should be investigated.
EPIDEMIOLOGY1,2
• Atrial fibrillation is the most common cardiac arrhythmia in clinical practice, accounting for approximately one-third of hospitalizations for cardiac rhythm disturbances.
• The estimated prevalence of AF is 0.4% to 1% in the general population, increasing with age.
• The age-adjusted prevalence of AF is higher in men.
• The median age of AF patients is about 75 years. Approximately 70% are between 65 and 85 years old.
• AF frequently accompanies common conditions such as hypertension, chronic heart failure, and valvular or ischemic heart disease, and is a common sequela of cardiothoracic surgery, especially in patients with valvular heart disease.
• The arrhythmia is associated with a five-fold risk of thromboembolic stroke and a three-fold incidence of congestive heart failure, and higher mortality.
• The risk of stroke is greater in the elderly and with concomitant valvular (particularly rheumatic) heart disease.
PATHOPHYSIOLOGY AND ETIOLOGY
• The etiology of AF is complex.
• AF is often an electrical manifestation of underlying cardiac disease. Nonetheless, approximately 30% to 45% of cases of paroxysmal AF and 20% to 25% of cases of persistent AF occur in younger patients without demonstrable underlying disease (“lone AF”).1
• Any kind of structural heart disease may trigger remodeling of both the atria and ventricles. Structural remodeling facilitates initiation and perpetuation of AF.
• The arrhythmia is characterized by disorganized electrical activity with multiple re-entrant circuits in the atria that can lead to electrical and structural remodeling of the atria, which in turn reinforces the establishment of AF.
• The most frequent pathoanatomic changes in AF are atrial fibrosis and loss of atrial muscle mass.
• Perhaps most significant is the discovery that a proportion of AF cases caused by triggering from foci within the pulmonary veins can be successfully ablated with radiofrequency treatment.
• AF is associated with delayed emptying from left atrial appendage resulting in reduced LAA flow velocities that are implicated in thrombus formation.
• Additionally, the left atrial appendage has become a focus of attention for surgical and percutaneous closure techniques that are becoming available.
ECHOCARDIOGRAPHY
• Almost all patients presenting with their first episode of AF will benefit from transthoracic echocardiography (TTE) evaluation of left atrial size, left ventricular systolic function, and mitral valve morphology and function.
• TTE provides detailed information about cardiac anatomy and function, but it is less useful for the detection of thrombus in the LA or LAA.
• Echocardiography has an increasingly important therapeutic role in guiding these ablation and LAA closure procedures.3
• The management of AF can be divided into rhythm versus rate control strategies, combined with a risk assessment for prevention of thromboembolism. Echocardiography plays a critical role in defining the clinical context of the arrhythmia and therefore informs the clinician regarding the key issues of anticoagulation and overall cardiac management.
• Transesophageal echocardiography (TEE) is not part of the standard initial investigation of patients with AF, but it is a sensitive and specific technique for detection of LA and LAA thrombus, compared to TTE. This modality also permits superior evaluation for other causes of cardiogenic embolism, as well as a means of measuring LAA function.
• This technology has been used to stratify stroke risk in patients with AF and to guide cardioversion.
• Several TEE findings have been associated with thromboembolism, including thrombus, reduced LAA flow velocity (≤20 cm/s), spontaneous echo contrast in the LA or LAA, and atheromatous disease of aorta.4
• Detection of LA/LAA thrombus stands as a contraindication to elective cardioversion (CV) of AF.
OTHER DIAGNOSTIC TESTING AND PROCEDURES
• Electrocardiogram (ECG) is the principal testing modality for the diagnosis and follow-up of AF.
• If the patient has normal sinus rhythm on ECG, but has suspected paroxysmal AF, Holter monitoring should be performed.
• An EP study can be helpful when AF is a consequence of reentrant tachycardia such as atrial flutter, intra-atrial reentry, or AV reentry involving an accessory pathway.
DIFFERENTIAL DIAGNOSIS
• Arrhythmias that can mimic AF include atrial tachycardia, atrial flutter with variable AV block, frequent atrial ectopies, and antegrade atrioventricular nodal conduction.
• Any episode of suspected AF should be recorded by a 12-lead ECG. An ECG recording will help in differentiating AF from rare supraventricular arrhythmias with irregular RR intervals.
• Occasionally, use of atrioventricular nodal blocking drugs, using Valsalva maneuver, carotid massage, or intravenous adenosine may be necessary to establish the diagnosis.
DIAGNOSIS
• An irregular pulse can increase the suspicion of AF, but ECG remains essential in diagnosing AF. AF is defined with the following characteristics:
![]() Irregular RR intervals.
Irregular RR intervals.
![]() No distinct P waves on ECG.
No distinct P waves on ECG.
![]() Atrial cycle length (interval between two atrial activations) is usually variable and <200 ms (>300 BPM).
Atrial cycle length (interval between two atrial activations) is usually variable and <200 ms (>300 BPM).
• Symptom-activated event recorders (30 day), Holter monitoring (24 h to 7 days), and implantable loop recorders can be used for AF diagnosis.
• AF may be categorized as paroxysmal (self-terminating), persistent (requiring electrical or pharmacological termination), or permanent (cardioversion impossible or futile).1
MANAGEMENT
• Management of patients with AF is directed at reducing symptoms by rate control, correction of rhythm disturbances, and prevention of thromboembolism. The management strategy should be discussed with the patient and should consider several factors:
• type and duration of AF
• severity and type of symptoms
• associated cardiovascular disease
• patient age
• associated medical conditions
• short-term and long-term treatment goals
• pharmacological and nonpharmacological therapeutic options.
• In a patient with asymptomatic persistent AF, attempts to restore sinus rhythm may not be needed.
• Prospective studies such as Rate Control Versus Electrical Cardioversion for persistent atrial fibrillation (RACE) and Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) showed that patients who tolerated rate-controlled AF had outcomes similar to those randomized to rhythm control.5,6
• None of the major trials demonstrated any significant difference in the quality of life with ventricular rate control compared to rhythm control.
• Drugs and ablation are effective for both rate and rhythm control, and in special circumstances, surgery may be the preferred option.
• For rhythm control, drugs are typically the first choice, and radiofrequency ablation is a second-line choice. In some patients, especially the young with very symptomatic lone AF who need sinus rhythm, radiofrequency ablation may be preferred over drug therapy.2
• Patients with preoperative AF undergoing cardiac surgery face a unique opportunity. While few patients are candidates for a stand-alone surgical procedure to cure AF using the MAZE procedure or LA ablation techniques, these approaches can be an effective adjunct to coronary bypass or valve surgery to prevent recurrent postoperative AF.
• Because the LAA is the site of over 95% of detected thrombi, this structure is often excluded from the circulation when possible during cardiac surgery in patients at risk of developing postoperative AF, although this has not been proven to prevent stroke.1
• Risk stratification for patients with nonvalvular AF is performed using the CHADS2 (Congestive heart failure, Hypertension, Age ≥75, Diabetes, Stroke [doubled]) risk assessment tool1 (Table 10-1-1).
• Another risk stratification schema is the CHA2DS2-VASc (Congestive heart failure/left ventricular dysfunction, Hypertension, Age ≥75 [doubled], Diabetes, Stroke [doubled]-Vascular disease, Age 65-74, and Sex category [female]) risk assessment tool.2
• Major risk factors are prior stroke, TIA, and older age (>75 years).
• Clinically relevant nonmajor risk factors include heart failure, moderate to severe systolic LV dysfunction (LVEF <40%), hypertension, age 65 to 74 years, diabetes, female sex, and vascular disease (MI, peripheral artery disease, complex aortic plaque).
• There is a clear relationship between the CHA2DS2-VASc score and stroke rate.
• In patients with a CHA2DS2-VASc score of 2 or more, chronic anticoagulation therapy is recommended unless contraindicated.
• An assessment of bleeding risk should be a part of the patient assessment before starting anticoagulation. It is reasonable to use the HAS-BLED score (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly (more than 65-years old), Drugs or alcohol use) to assess the bleeding risk in atrial fibrillation patients.
TABLE 10-1-1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


