SECTION 1
CLINICAL CASE PRESENTATION
A 50-year-old man presented to the Ohio State University Medical Center for evaluation of elevated blood pressures noted at a routine eye appointment. He had been told of high blood pressures for at least the last 5 years, but he had not been established with a primary physician at the time and did not seek out medical attention. He recently became established with a primary physician and was noted to have a blood pressure greater than 170/110 mm Hg on repeat evaluations. He was on no medications at the time and otherwise denied any symptoms. His exam was normal other than the elevated blood pressure and a faint S4 gallop heard on exam. His creatinine was mildly elevated (1.4 mg/dL), and an ECG showed borderline LVH by voltage criteria. He was reluctant to start any medications and was referred for an echocardiogram. This revealed moderate concentric hypertrophy of the left ventricle (Figures 12-1-1 and 12-1-2), a mildly dilated aortic root (Figure 12-1-3), left atrial enlargement (Figure 12-1-4), and grade II diastolic dysfunction (Figures 12-1-5 and 12-1-6). He agreed to start medical therapy and subsequent blood pressures have been normal at clinical visits.
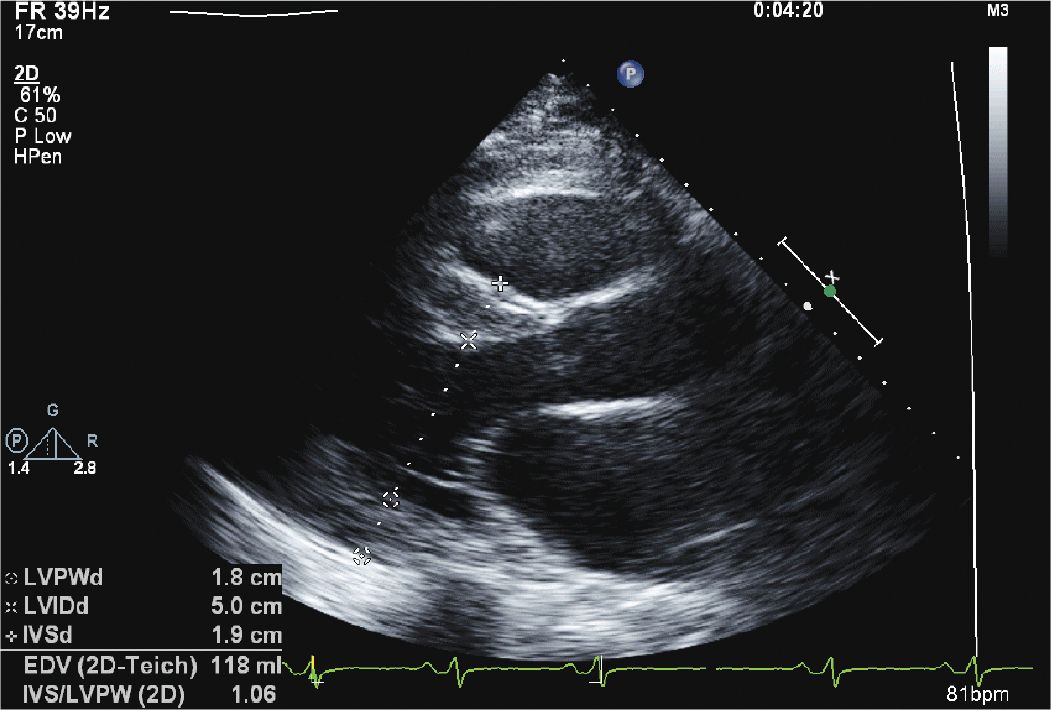
FIGURE 12-1-1 Parasternal long axis view of the left ventricle showing concentric hypertrophy of the left ventricle. The septal thickness measures 1.9 cm with a posterior wall thickness of 1.8 cm.

FIGURE 12-1-2 Parasternal short axis view demonstrating concentric left ventricular hypertrophy.
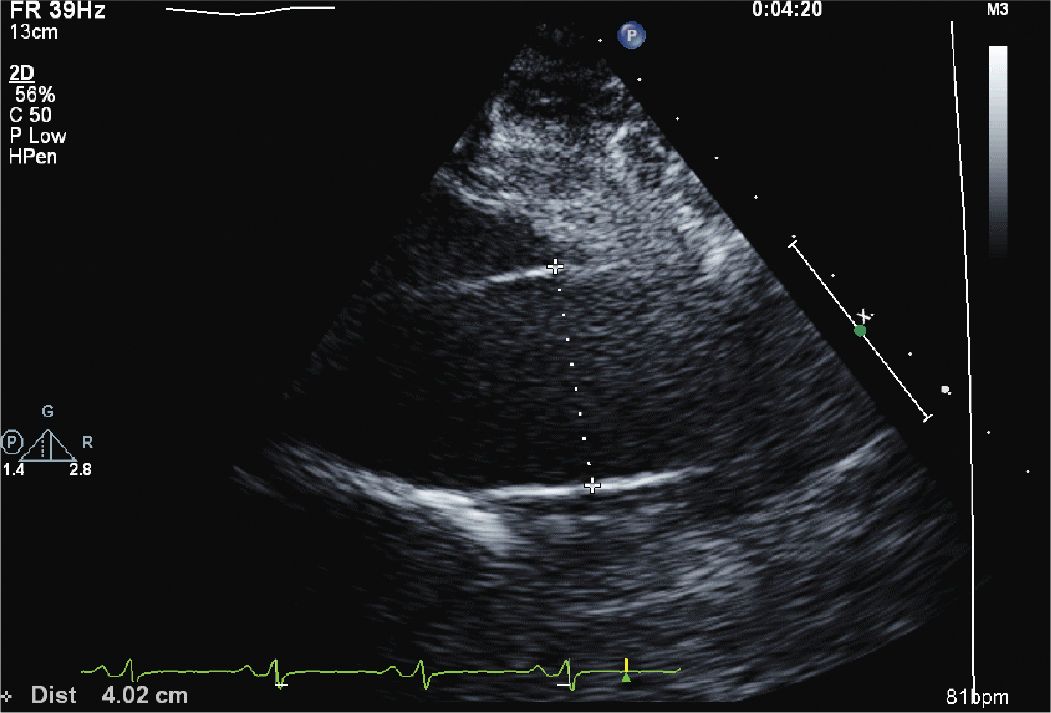
FIGURE 12-1-3 Parasternal view of the ascending aorta, which is mildly dilated (4.0 cm).
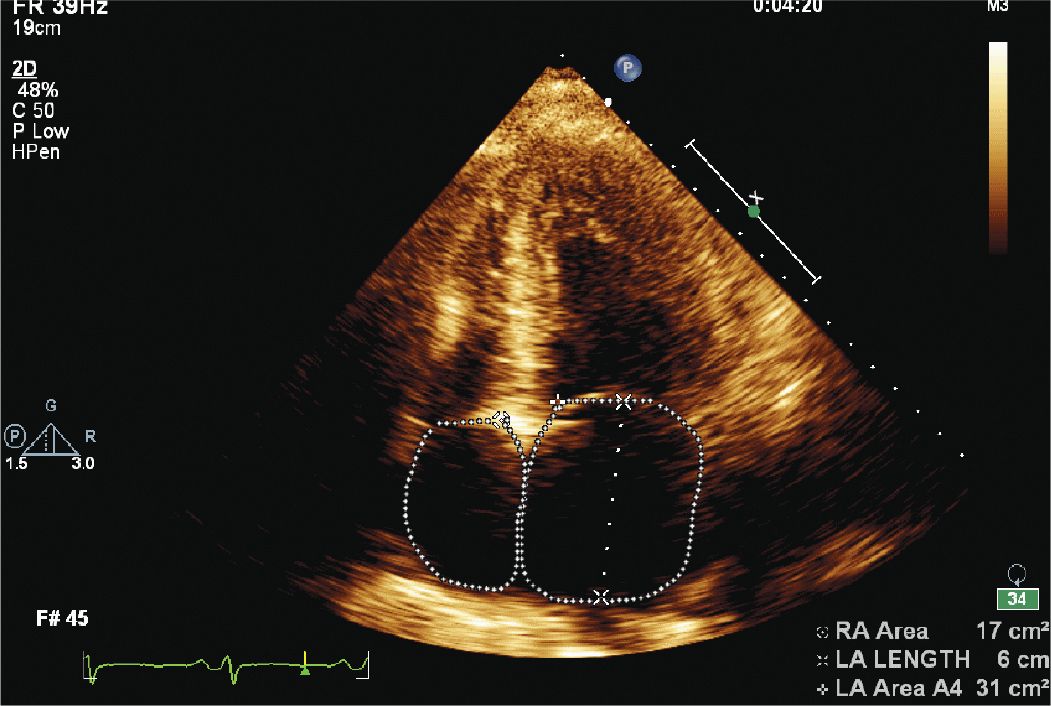
FIGURE 12-1-4 Apical 4 chamber view showing left atrial enlargement.
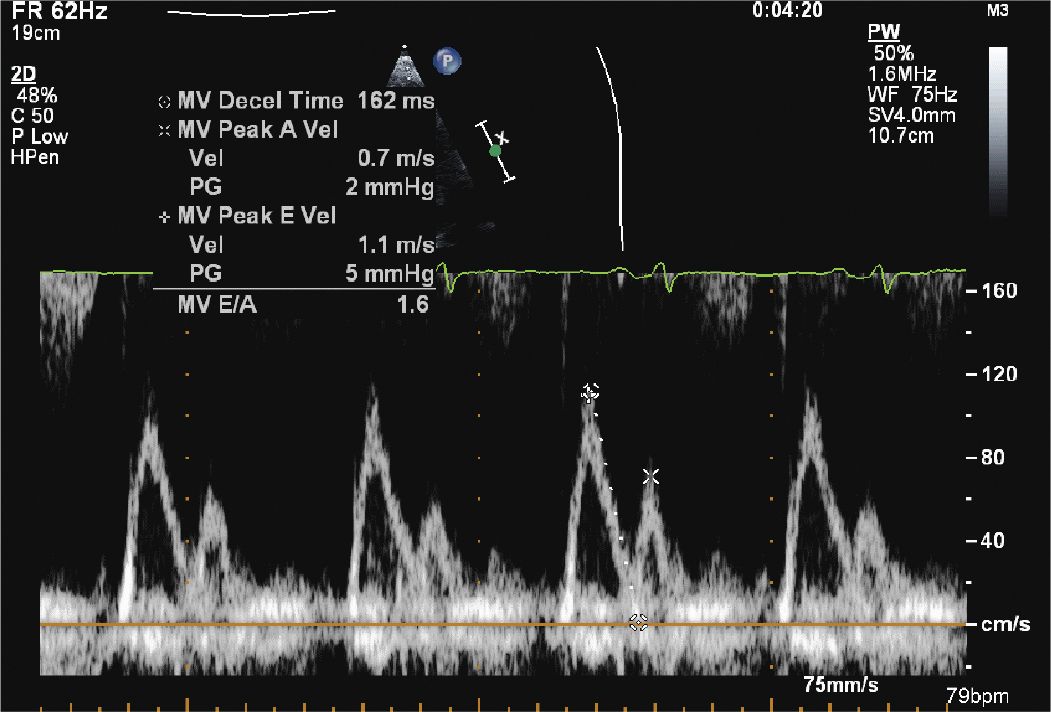
FIGURE 12-1-5 Mitral inflow velocity with a normal E/A ratio; however, the deceleration time is somewhat fast. This, in combination with the TDI (Figure 12-1-6) suggests moderate diastolic dysfunction.
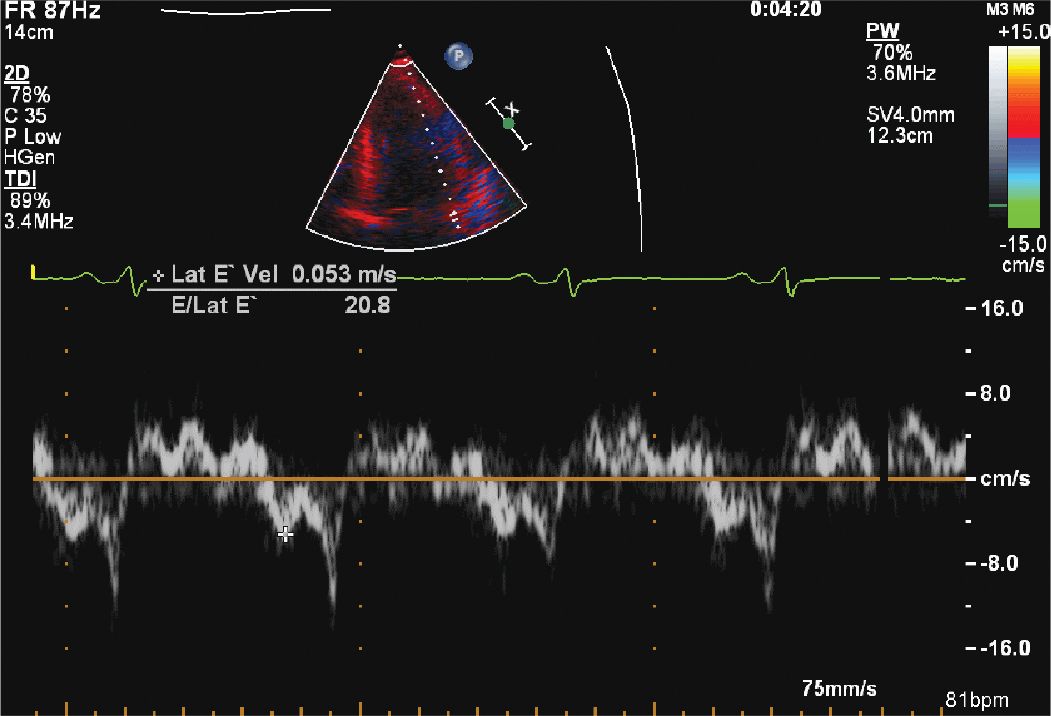
FIGURE 12-1-6 Tissue Doppler of the lateral mitral annulus with an elevated E/e’ ratio, suggesting moderate diastolic dysfunction.
CLINICAL FEATURES
Hypertension, for the most part, is an asymptomatic disease in the early stages and is easily detected with routine screening. With prolonged hypertension, however, patients may show signs of end organ damage including renal failure, retinopathy, strokes, vascular disease, or progressive heart failure.
• In hypertensive heart disease, the early stages show signs of left ventricular hypertrophy including an S4 gallop due to the noncompliant nature of the left ventricle (LV).
• As the disease progresses, signs of heart failure and volume overload can be present, including peripheral edema or pulmonary congestion.
• In some patients, the LV further remodels and dilates producing signs of systolic heart failure with an S3 gallop and a laterally displaced apical impulse.
• Other signs of end-organ damage should be sought as well, including hypertensive retinopathy, neurologic defects, renal artery bruits, or an abdominal aneurysm.
EPIDEMIOLOGY
Hypertension is a common clinical entity and may be present in up to 28% of the population of North America and 44% in Europe.1 Left ventricular hypertrophy (LVH) is also often seen during routine clinical echocardiograms (15%). Furthermore, an increase in blood pressure is associated with an increased prevalence of LVH—up to 60% in patients with severe hypertension.2 Hypertension is an independent risk factor for coronary disease, heart failure, renal failure, and stroke. LVH as well, is associated with an increase in cardiovascular morbidity including sudden death, heart failure, and mortality with a relative risk of 2.08 compared to patients with normal LV mass.3
PATHOPHYSIOLOGY AND ETIOLOGY
• LVH is at first an adaptive response to the load placed on the heart, in particular, increased afterload. Wall thickness and hypertrophy serve to decrease the overall LV wall stress and myocardial oxygen demand.
• Multiple hemodynamic, genetic, and neurohormonal factors likely contribute to the overall process of myocyte hypertrophy.
• Over time, however, this increase in left ventricular mass leads to diastolic dysfunction and increasing myocardial fibrosis.4
• In some patients, there is further remodeling, including cell apoptosis, eccentric remodeling, and eventually, systolic heart failure.
• In addition to the effects on the myocardium, hypertension affects endothelial function and vascular stiffness.
• Aortic dilation with or without aortic valve insufficiency can be a common consequence of hypertension.
• Hypertension can increase the risk of coronary vascular disease and myocardial ischemia with both macrovascular and microvascular consequences.
• All of these effects serve to increase the risk of overall cardiac morbidity and mortality3 making this an important diagnosis to make at an early stage.
ECHOCARDIOGRAPHY
One of the earliest and most recognizable consequences of hypertensive heart disease is the increase in left ventricular mass.
• The American Society of Echocardiography (ASE) recommends calculating the LV mass based on the 2-D directed measurement of the septal wall thickness (SWTd), posterior wall thickness (PWTd), and the end diastolic cavity dimension (LVIDd).5 The left ventricular cavity area is subtracted from the overall LV area using the equation:
![]()
• A left ventricular mass index (gm/M2) greater than 95 in women and 115 in men suggests LV hypertrophy.
• The relative wall thickness (RWT), defined as (2 × PWTd)/LVIDd helps to further classify the LV hypertrophy as either eccentric (RWT<0.42) or concentric (RWT>0.42). These two-dimensional (2-D) methods have the disadvantage of assuming a uniform shape to the heart. In the cases of uniform remodeling, this is typically accurate. However, with deformities in LV geometry, this assumption becomes less accurate.
• Some laboratories have begun to use three-dimensional echocardiographic techniques, which have been shown to correlate well with other techniques including cardiac MRI.
Diastolic dysfunction is another effect of ventricular hypertrophy and remodeling and can be evaluated by the combination of mitral inflow velocities, tissue Doppler, and secondary effects including left atrial enlargement, pulmonary vein flow patterns, and elevated right ventricular pressures.
• The ratio of the early to late mitral inflow velocities (E/A ratio) may be the first sign of hypertensive heart disease even in the absence of LVH.
• The ratio of the early mitral inflow velocity (E) to the early mitral annulus velocity (e’) can be used to estimate LV filling pressures.6
• Newer techniques to evaluate strain and strain rate of the myocardium may in the future help better define this process as well.
In addition to abnormalities of the left ventricle, patients with hypertensive heart disease can show vascular changes including aortic aneurysms or dilation of the proximal ascending aorta. This may result in aortic insufficiency due to issues with valve coaptation.
Valve thickening or mitral annular calcification can also be seen, especially in patients with concomitant renal disease.
OTHER DIAGNOSTIC TESTING AND PROCEDURES
Secondary causes of hypertension should be sought in selected patients and may include renal disease (both as a cause and a consequence of hypertension), endocrine disorders, coarctation of the aorta, and drugs.
• Laboratory evaluations should be guided by the history and physical exam as well as clinical suspicion.
• An ECG is vital in screening patients with hypertension and can detect chamber enlargement and conduction system disease such as a left bundle branch block or atrial fibrillation.
• The chest x-ray can be helpful in patients presenting with shortness of breath and signs of pulmonary edema.
• If an echocardiogram is insufficient to estimate left ventricular function or dimensions, a cardiac MRI can accurately measure LV function and mass as well as provide evidence of myocardial fibrosis.
• With the prevalence of coronary disease in patients with long-standing hypertension, stress testing can also be used if the clinical scenario dictates.
DIFFERENTIAL DIAGNOSIS
Left ventricular hypertrophy due to hypertensive heart disease, especially in extreme cases, can be difficult to distinguish from other causes of LV hypertrophy including hypertrophic cardiomyopathy, Fabry disease, and athlete’s heart. With progressive remodeling, thinning of the ventricle and chamber enlargement, the end stage of hypertensive heart disease can be difficult to distinguish from other cause of dilated cardiomyopathies.
DIAGNOSIS
• The diagnosis of hypertensive heart disease with echocardiography is made by demonstrating changes in the left ventricle, including increased LV mass or diastolic dysfunction with or without ECG changes consistent with LVH.
• Other potential causes of left ventricular hypertrophy should be ruled out as well before a presumptive diagnosis is made in patients with hypertension.
• Several symptoms can also be attributed to hypertensive heart disease, including exertional chest pain or shortness of breath, and are related to microvascular disease or increased myocardial oxygen demand. These should be distinguished from other causes, especially epicardial coronary disease, as these conditions share many of the same risk factors.
MANAGEMENT
The main treatment for hypertensive heart disease is aggressive lowering of the systemic blood pressure. The Joint National Committee (JNC 7) guidelines provide a framework for clinical control of blood pressure and selection of initial blood pressure agents.7 Treating blood pressure in patients with left ventricular hypertrophy is particularly important as a reduction in left ventricular mass has been associated with reduction of cardiac events.8 Selected medications, particularly ACE inhibitors, angiotensin receptor blockers, and calcium channel blockers—may have a faster effect on the rate at which left ventricular mass decreases; however, the clinical implications of this are not well established.9 Patients should be followed to ensure that their resting blood pressures are within standard targets (<130/80) as well as any age-appropriate screening or signs of other end organ damage. Echocardiograms can be performed as symptoms dictate. If heart failure develops, these patients should be treated with standard heart failure regimens.
REFERENCES
1. Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289(18):2363-2369.
2. Levy D, Anderson KM, Savage DD, et al. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108(1):7-13.
3. Verdecchia P, Carini G, Circo A, et al. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol. 2001;38(7):1829-1835.
4. Raman SV. The hypertensive heart. An integrated understanding informed by imaging. J Am Coll Cardiol. 2010;55(2):91-96.
5. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79-108.
6. Nagueh SF, Appelton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165-193.
7. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206-1252.
8. Pierdomenico SD, Cuccurullo F. Risk reduction after regression of echocardiographic left ventricular hypertrophy in hypertension: a meta-analysis. Am J Hypertens. 2010;23(8):876-881.
9. Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003;115(1):41-46.
SECTION 2
CLINICAL CASE PRESENTATION
A 45-year-old woman presented to the hospital with increasing shortness of breath. She had a longstanding history of diabetes and hypertension, which had resulted in renal failure, and had undergone a renal transplant 3 years prior to admission. Her renal function had gradually declined over the course of the last year, and over the last several weeks, she had been having dyspnea, fatigue, and worsening lower extremity edema. On admission, she was breathing comfortably while in bed. She was hypertensive with a blood pressure of 170/100 mm Hg. Her cardiac exam revealed a systolic ejection murmur with brisk carotid upstrokes. Jugular venous distension was noted to the angle of the jaw at 45°. She had rales in the bases of her lungs, and 2+ edema was noted in the lower extremities bilaterally. Her BUN was 90 mg/dL, and her creatinine was 4.80 mg/dL (her baseline had been approximately 2.0). Her chest x-ray showed mild pulmonary edema. Her echocardiogram showed mild left ventricular hypertrophy (LVH) (Figures 12-2-1 to 12-2-3) with calcification of the aortic and mitral leaflets without significant stenosis (Figures 12-2-4 and 12-2-5). She had mitral annular calcification as well as an enlarged left atrium (Figure 12-2-6). A small- to moderate-sized pericardial effusion was also noted without any signs of tamponade (there was no significant mitral inflow variation, no evidence of RA or RV collapse and 50% collapse of the IVC with respiration) (Figures 12-2-7 to Figure 12-2-9). She was initiated on dialysis with resolution of her symptoms and plans were made for permanent dialysis.
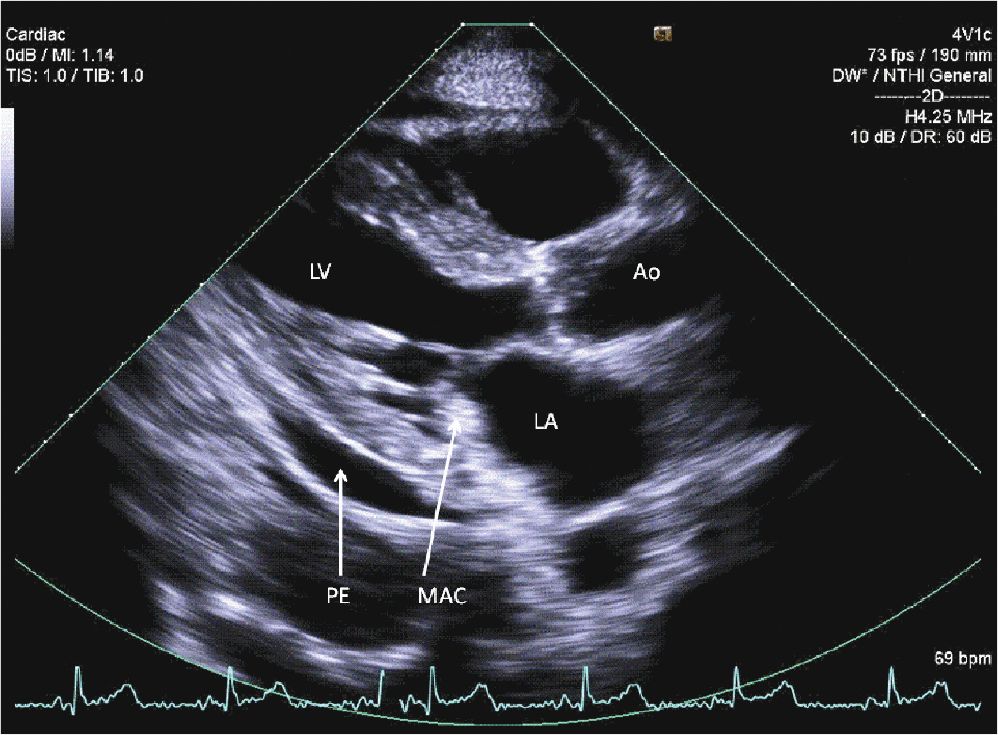
FIGURE 12-2-1 Parasternal long axis view of the heart. Note the mitral annular calcification (MAC) along with a small pericardial effusion (PE).
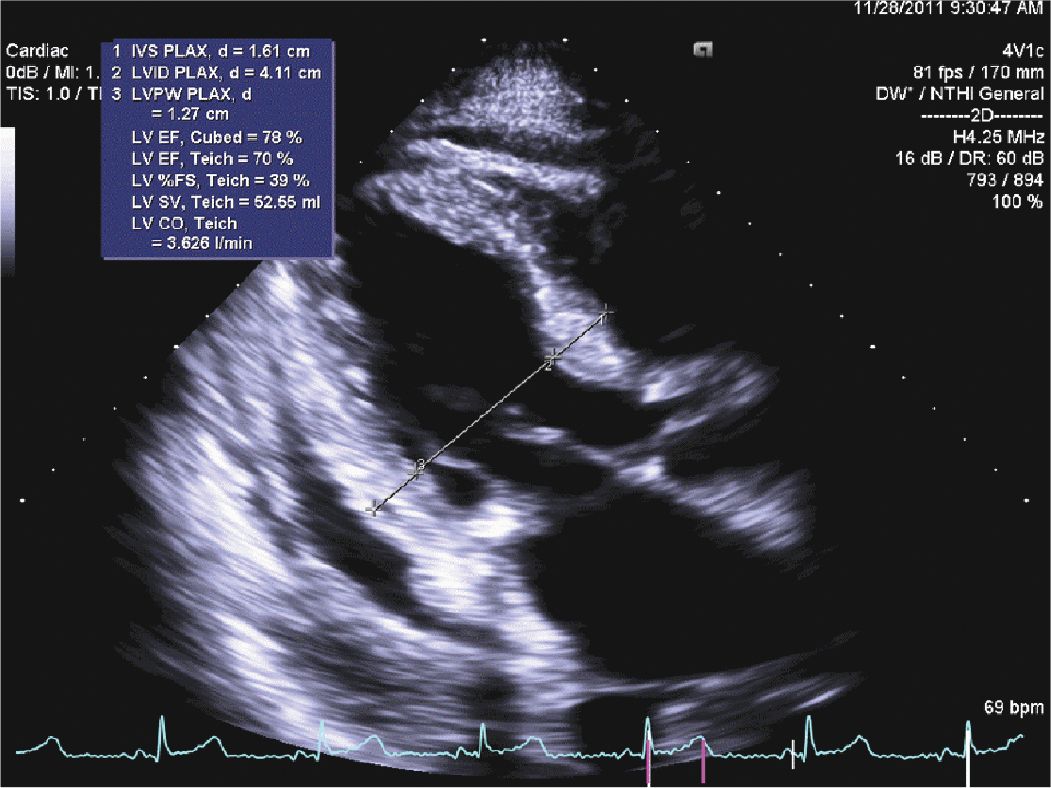
FIGURE 12-2-2 Concentric hypertrophy of the left ventricle is noted with normal LV chamber size. The septum measures 1.6 cm, and the posterior wall measures 1.3 cm.
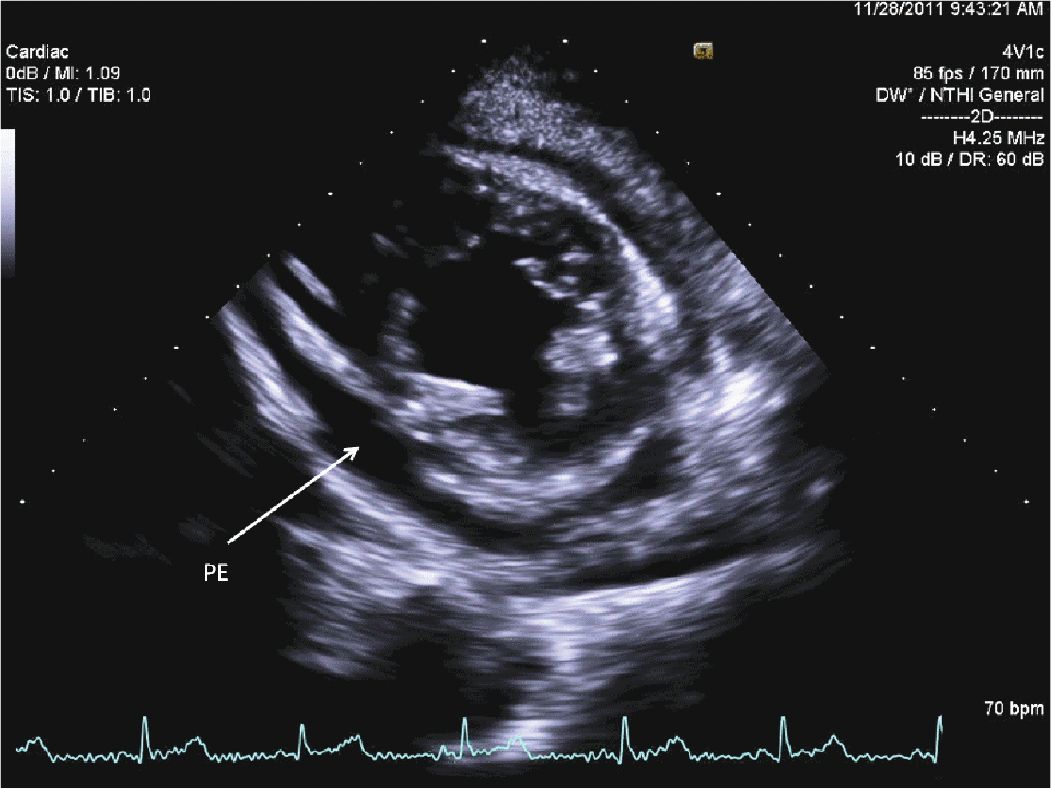
FIGURE 12-2-3 Parasternal short axis view showing LVH and a circumferential pericardial effusion (PE).
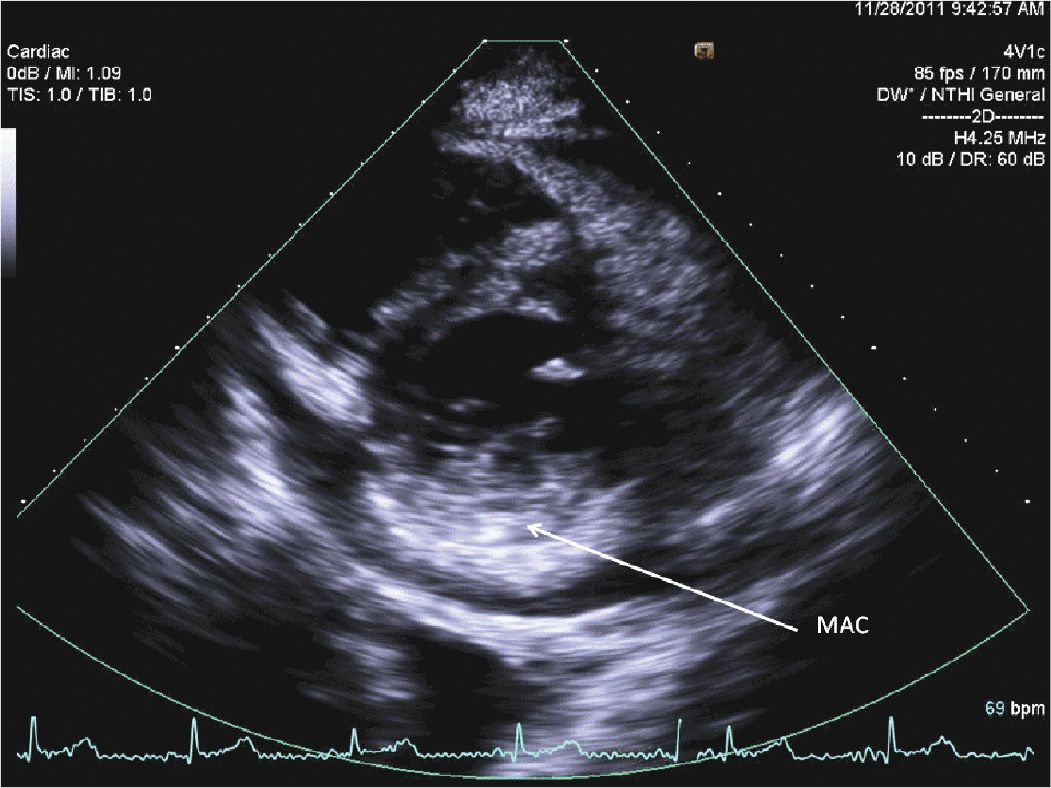
FIGURE 12-2-4 Parasternal short axis view of the mitral valve showing mitral annular calcification (MAC).
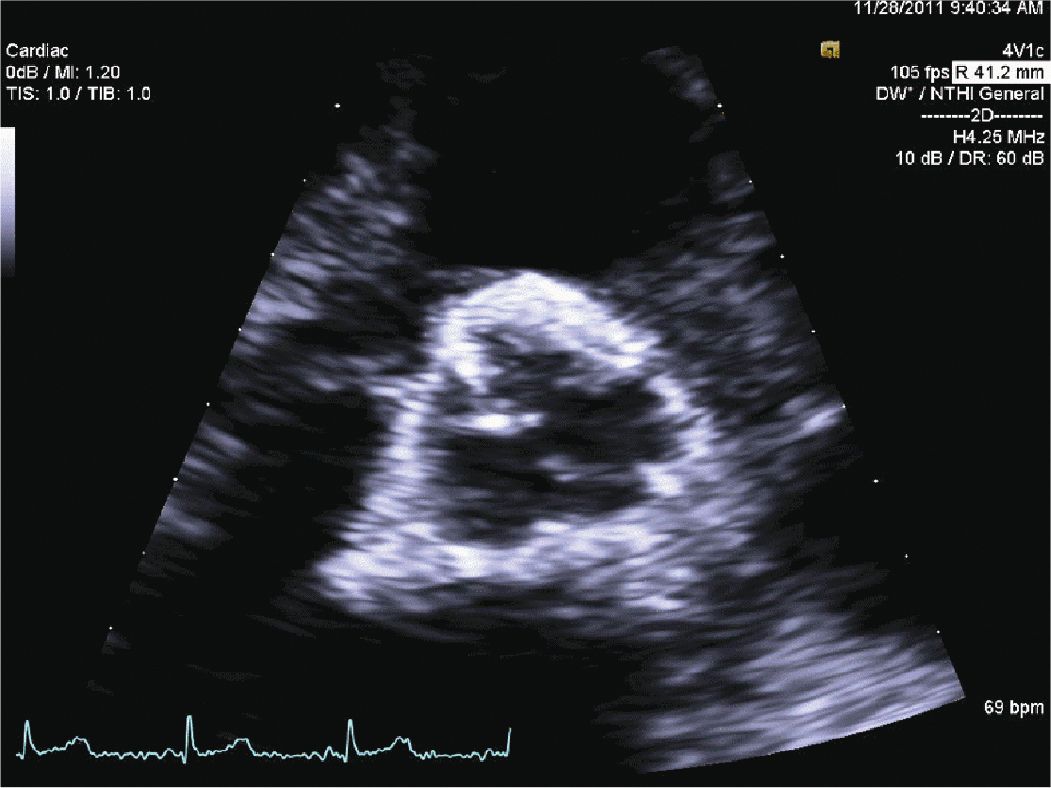
FIGURE 12-2-5 Parasternal short axis view of the aortic valve.
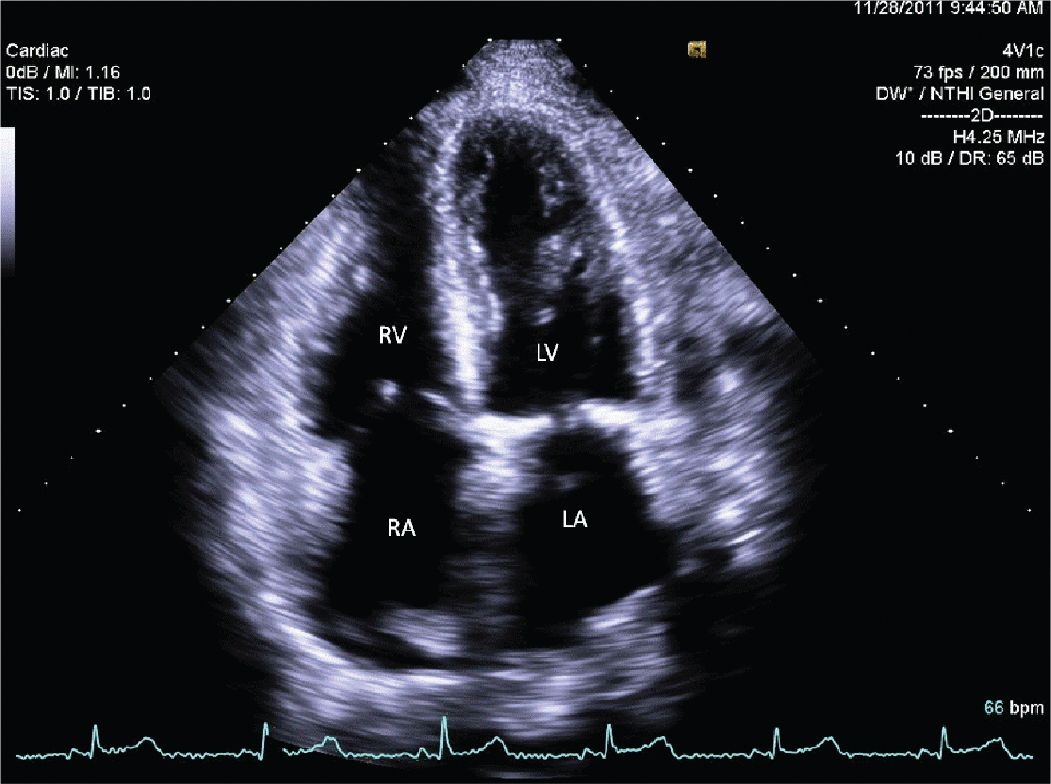
FIGURE 12-2-6 Apical 4 chamber view showing biatrial (LA and RA) enlargement.
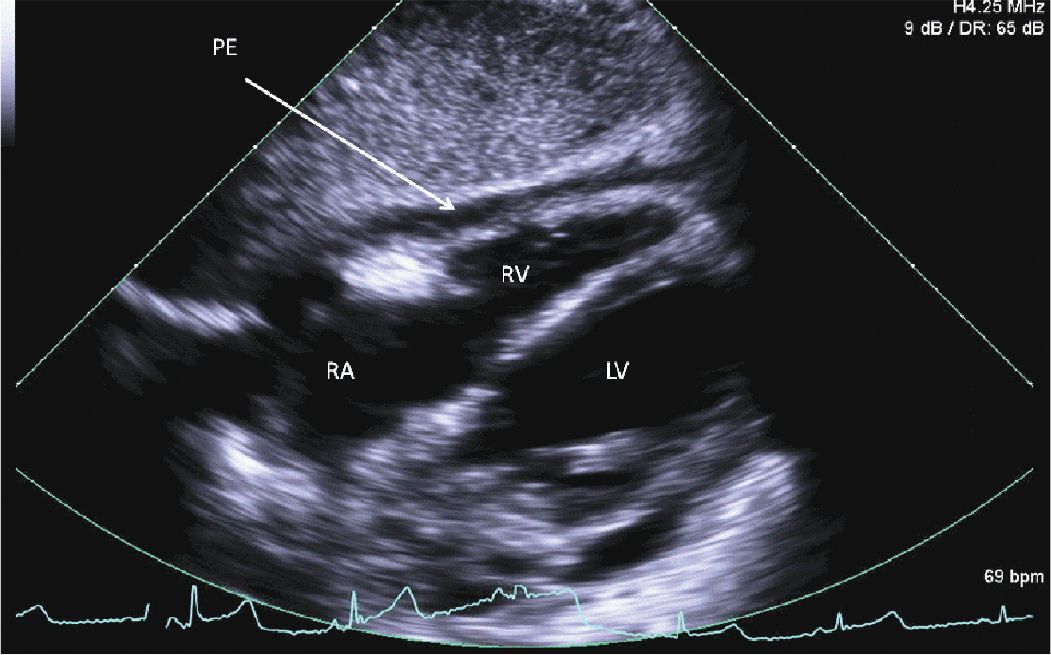
FIGURE 12-2-7 Subcostal 4 chamber view with a circumferential pericardial effusion (PE) and no evidence of RV or RA collapse.
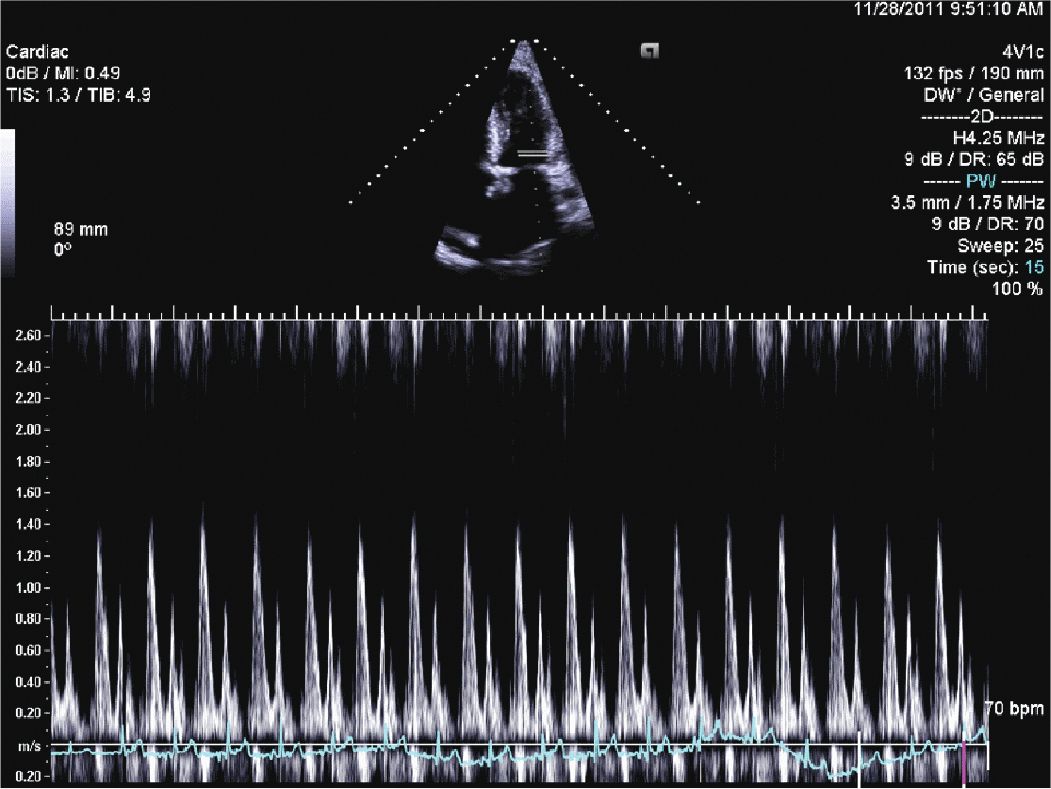
FIGURE 12-2-8 Mitral valve inflow showing no significant variation with respiration.
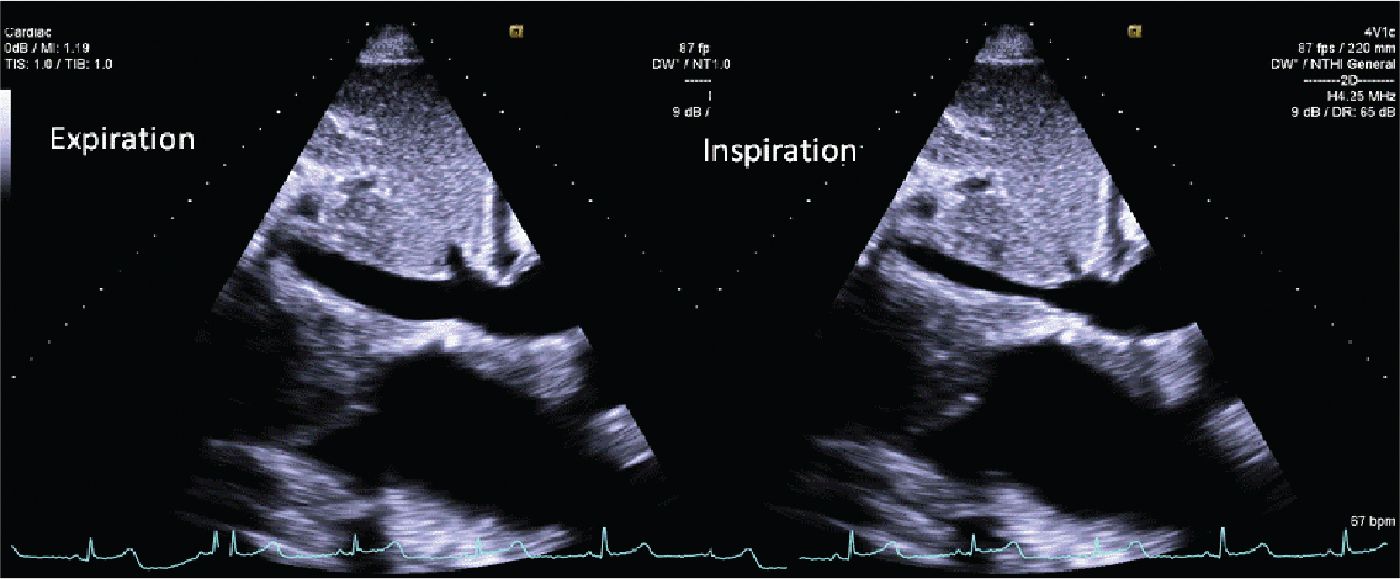
FIGURE 12-2-9 Subcostal view of the inferior vena cava during expiration (L) and inspiration (R). Overall, there is IVC dilation, but with >50% variation during inspiration.
CLINICAL FEATURES
• Renal disease can affect the heart in multiple ways, from valvular heart disease to myocardial infarction, heart failure, and pericardial tamponade. There is significant overlap of risk factors for these conditions, and multiple issues can present in the same patient. Heart failure is one common presentation.
• Either dilated cardiomyopathies with low output heart failure or significant diastolic heart failure can be present with the latter often being difficult to distinguish from worsening renal function with volume retention.
• Ischemic heart disease is also common in this population with myocardial infarction and ischemic cardiomyopathy being some of the most frequent causes of morbidity and mortality in these patients.
• Valvular heart disease typically presents with increasing calcification of the valves leading to eventual valvular stenosis in some patients.
• Endocarditis is a particular concern in dialysis patients or patients with chronic indwelling catheters and can lead to further valve dysfunction.
• Finally, pericardial disease can present with a uremic pericarditis, including a friction rub or pericardial effusion of varying degrees.
EPIDEMIOLOGY
• Cardiac disease accounts for significant morbidity and mortality in patients with renal failure. In fact, renal patients are 30 times more likely to die from cardiovascular disease than the general population.1
• Coronary vascular disease is particularly common, but heart failure, valvular disease, and arrhythmias are also more common in this population, not to mention acute illnesses such as pericarditis and endocarditis. Valvular thickening and calcification can occur in up to 60% of patients on chronic dialysis with increased risk of valvular stenosis as well.2
• Given this high prevalence, aggressive risk factor modification and screening are important along with a heightened suspicion of cardiac disease.
PATHOPHYSIOLOGY AND ETIOLOGY
• Part of the pathophysiology of cardiac disease in this population certainly comes from the overlap of traditional cardiovascular disease risks factors and those for the development of renal disease. Chief among these are vascular disease, diabetes, and hypertension.3
• The accelerated nature of cardiovascular disease also accounts for an increased prevalence of heart failure and ischemic cardiomyopathy.
• These risk factors alone, however, do not account for all of the increased risk of cardiac diseases seen in this population.
![]() Several potential etiologies have been proposed including abnormalities in calcium metabolism, inflammation, and uremia.
Several potential etiologies have been proposed including abnormalities in calcium metabolism, inflammation, and uremia.
![]() Increases in calcium levels and secondary hyperparathyroidism have been associated with early calcification of the mitral and aortic valves.2
Increases in calcium levels and secondary hyperparathyroidism have been associated with early calcification of the mitral and aortic valves.2
![]() Finally, the presence of indwelling catheters and vascular devices increase the risk of infections and endocarditis.
Finally, the presence of indwelling catheters and vascular devices increase the risk of infections and endocarditis.
ECHOCARDIOGRAPHY
• Aside from diagnosing potential causes of renal failure (amyloidosis or systolic heart failure for example), multiple echocardiogram findings have been associated with patients on dialysis or with chronic kidney disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


