SECTION 1
CASE PRESENTATION
A 57-year-old man with a history of lung cancer and severe COPD presented to our institution with progressive dyspnea and dizziness. He denied any chest pain, recent febrile illness, cough or other constitutional symptoms other than anorexia. On arrival to the ED, he was noted to be tachypneic and tachycardic. His BP was 90/60 mm Hg. His lung exam revealed decreased breath sounds bilaterally, more prominent on the right. His neck veins were elevated. Cardiac exam revealed no murmurs; however, his heart sounds were distant. He had no edema. A chest x-ray revealed cardiomegaly and a right-sided pleural effusion. A stat bedside TTE revealed a large pericardial effusion with evidence of cardiac tamponade. He was taken to the cardiac catheterization lab where he underwent an echo-guided pericardiocentesis (Figures 13-1-1 to 13-1-3). Postprocedure echocardiography revealed resolution of the effusion. He was admitted to the oncology service. The pericardial drain was removed 2 days later after a limited echo revealed no reaccumulation of the fluid. He underwent further treatment of his lung cancer.
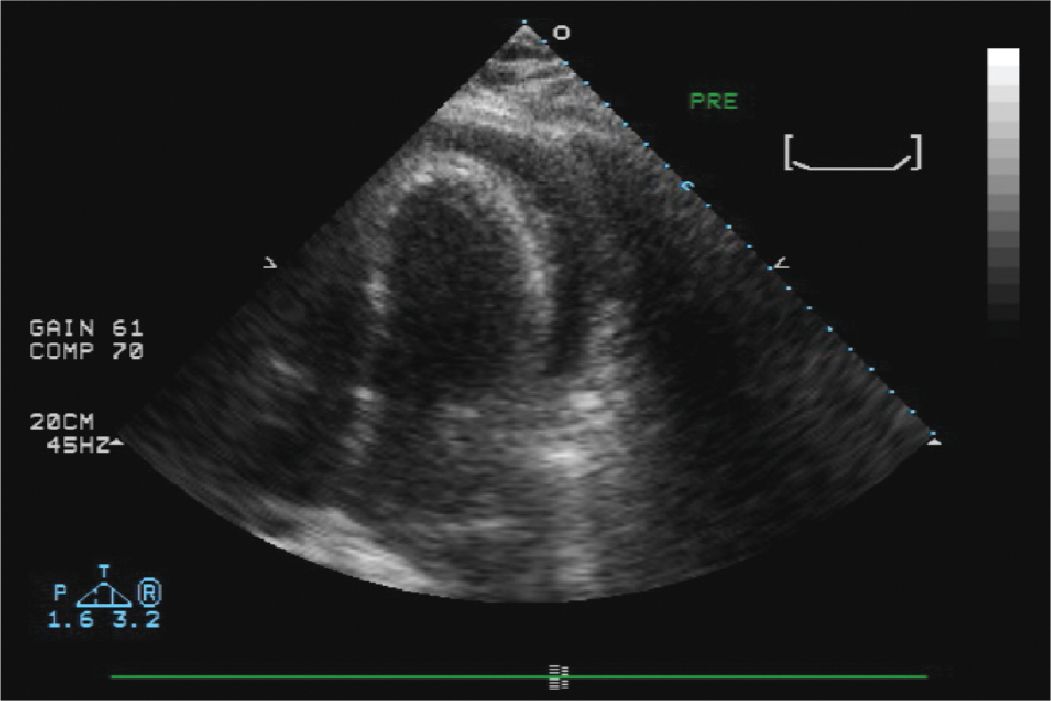
FIGURE 13-1-1 Initial echocardiogram in the catheterization lab which confirmed the presence of a large pericardial effusion.
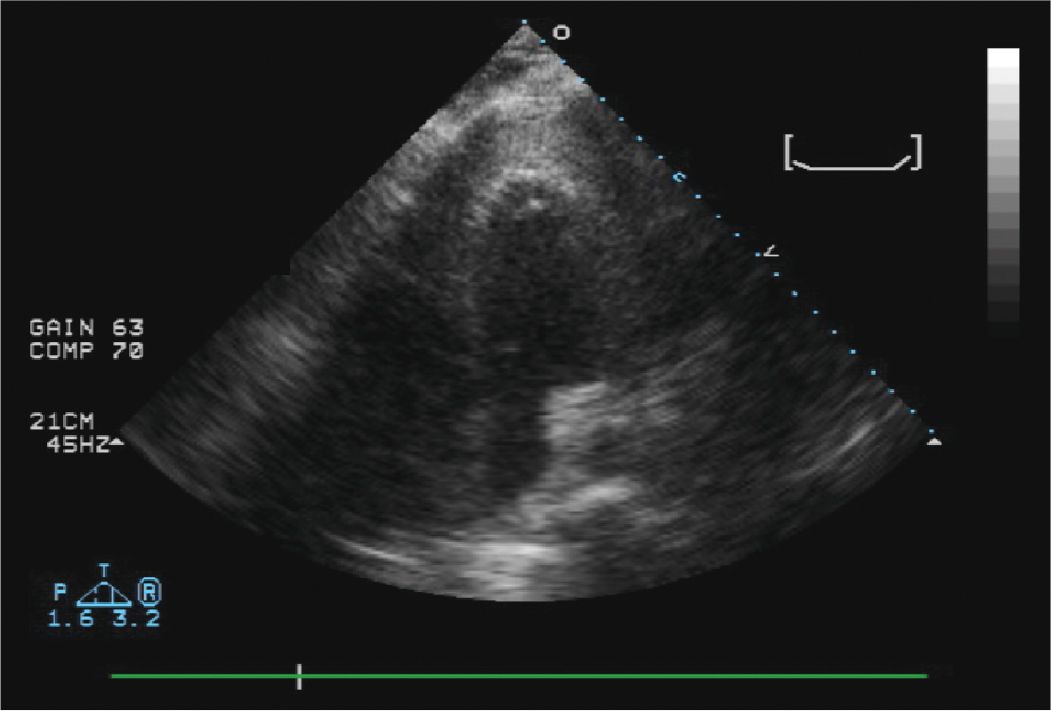
FIGURE 13-1-2 Apical 4 chamber view from the procedure. Agitated saline was injected through the needle to confirm that the tip of the needle was indeed in the pericardial space and not in the right ventricle. The “hazy” echoes at the LV apex are the saline contrast “bubbles”.
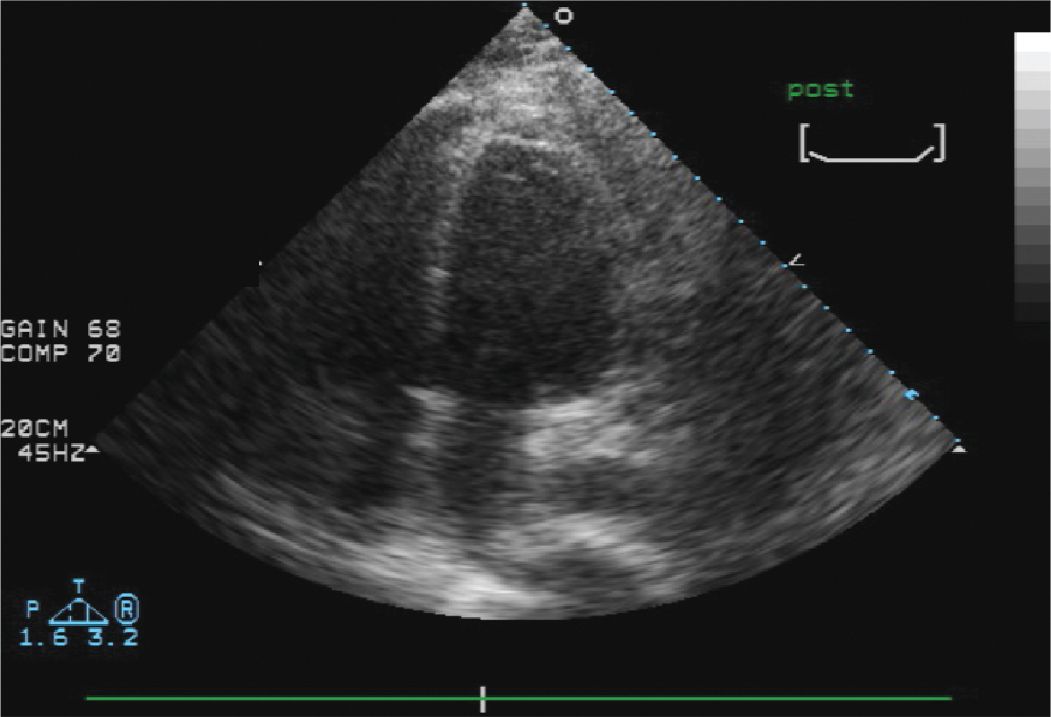
FIGURE 13-1-3 Post-tap echo demonstrating that the effusion is no longer present. The pericardium does appear thickened.
SECTION 2
CASE PRESENTATION
A 72-year-old man with a history of permanent atrial fibrillation and a CHADS2 score of 3 has been maintained on chronic anticoagulation. He has a history of congestive heart failure, hypertension, Type 2 diabetes, and peripheral vascular disease. While on warfarin, with a therapeutic INR, he developed a spontaneous subdural hematoma. His coagulopathy was reversed with Vitamin K, and he underwent successful evacuation of the hematoma. He recovered with minimal neurologic sequelae. After discussion with the neurosurgical team and the patient, it was determined that systemic anticoagulation was not advisable. The patient was approached about LAA isolation with the LARIAT device, a new procedure that is performed to exclude the LAA from the body of the LA. The LAA is the site of thrombus formation in the majority of patients with atrial fibrillation. Exclusion of the LAA hopefully will decrease the patient’s risk of stroke. The device was successfully placed with no residual communication between the LA and the LAA. He was discharged from the hospital on aspirin and his other cardiac medications and has done well with no systemic embolic events to date. The device is placed using a delivery system, which incorporates a guiding catheter system placed into the LAA via a transseptal puncture from the RA into the LA, and a delivery system for the LARIAT suture device placed externally around the LAA, which is delivered via the pericardial space.
TEE was used in this patient’s procedure to exclude the presence of a preexisting thrombus (a contraindication to the procedure), to guide device placement, and exclude complications such as pericardial effusion. At the end of the procedure, TEE imaging confirmed that there was no residual communication between the LA and the LAA. Figures 13-2-1 to 13-2-6 are representative images from his procedure. He has done well with no neurologic events. A follow-up TEE subsequently confirmed that there was no communication between the occluded LAA and the LA, nor was there any thrombus at the site of the LAA closure.
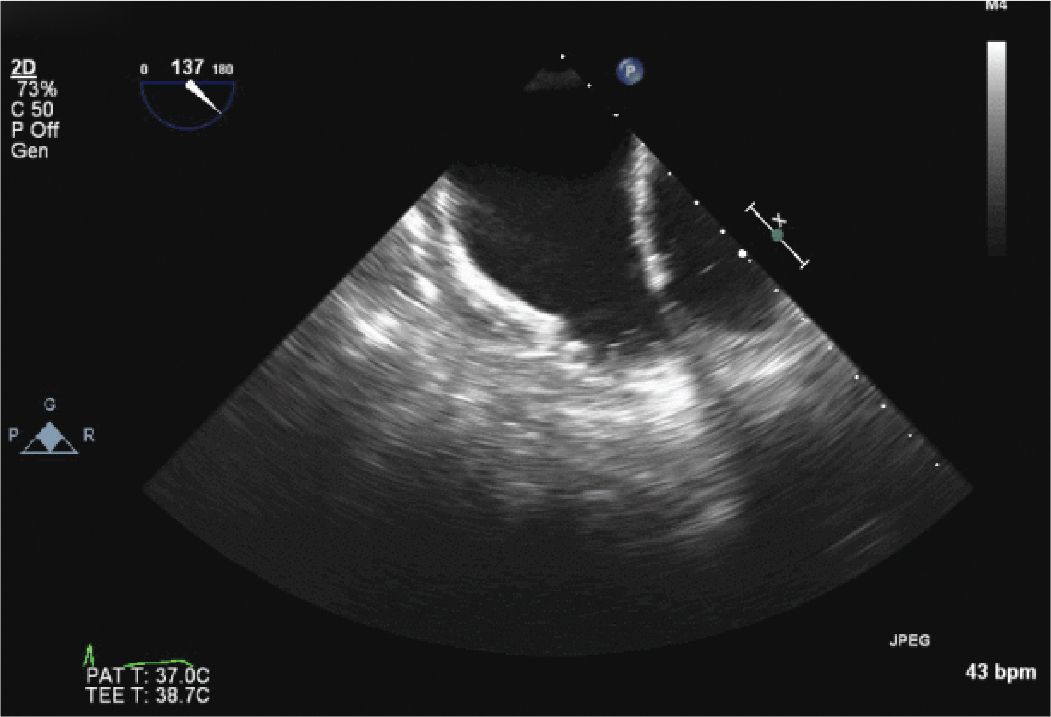
FIGURE 13-2-1 Initial TEE images of the LAA confirming the absence of an LAA thrombus. The ridges seen along the distal LAA wall are pectinate muscles, not thrombus.
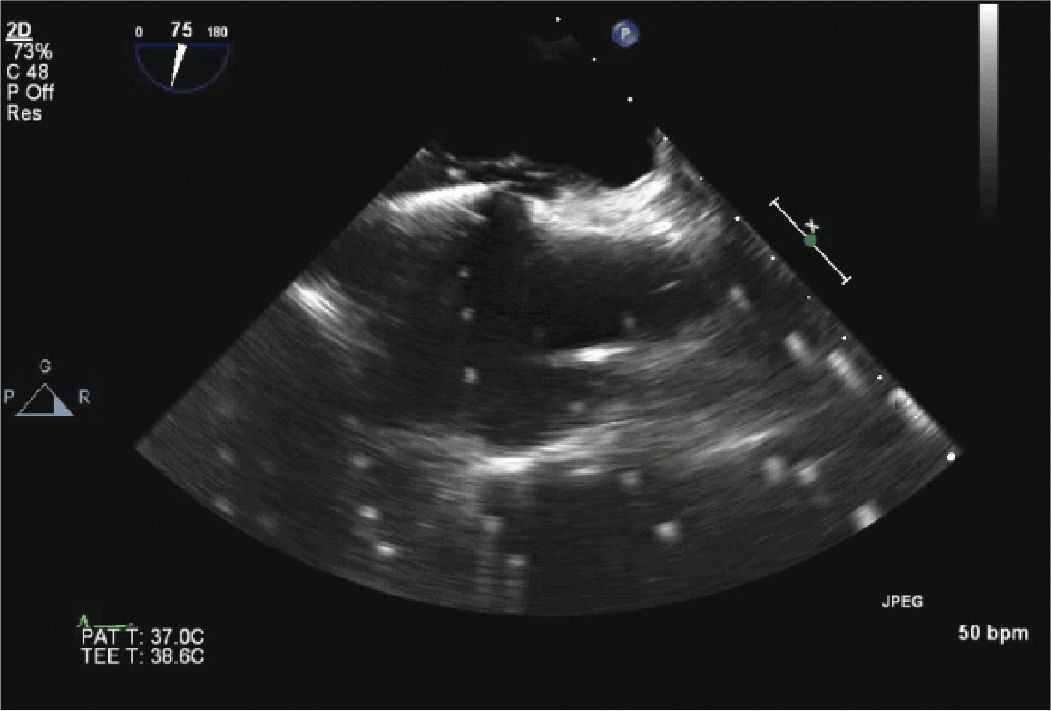
FIGURE 13-2-2 Transseptal Puncture. The catheter is being placed across the interatrial septum. The bright flashing signals are due to artifact from an intracardiac echo probe.
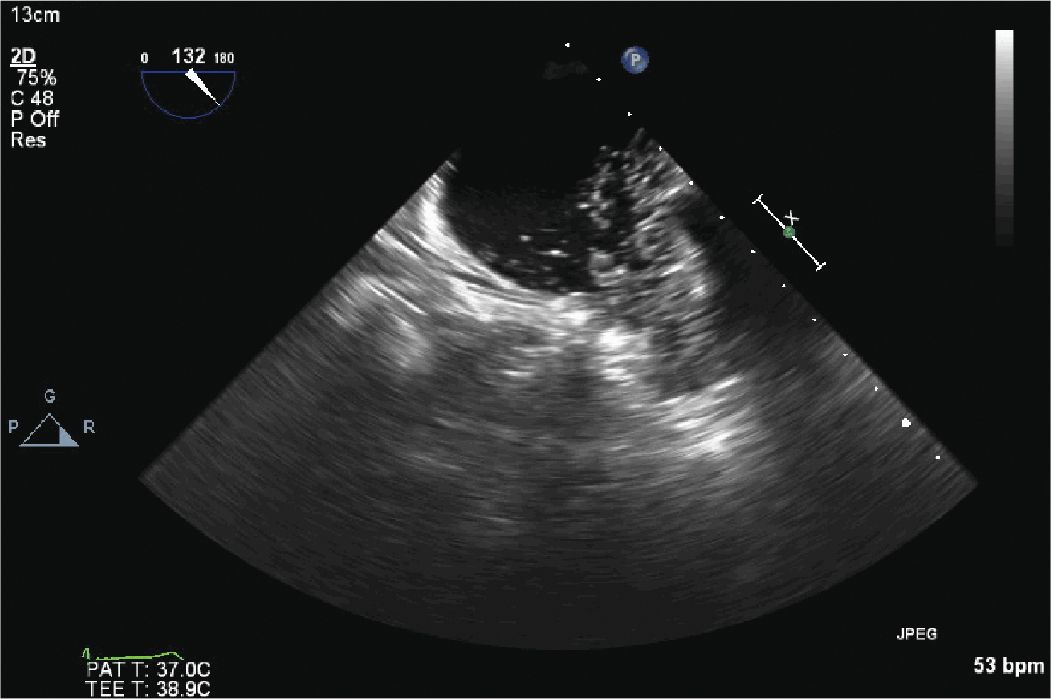
FIGURE 13-2-3 Contrast is injected into the LAA through the catheter in the LAA to define the LAA anatomy and confirm placement of the endocardial catheter.
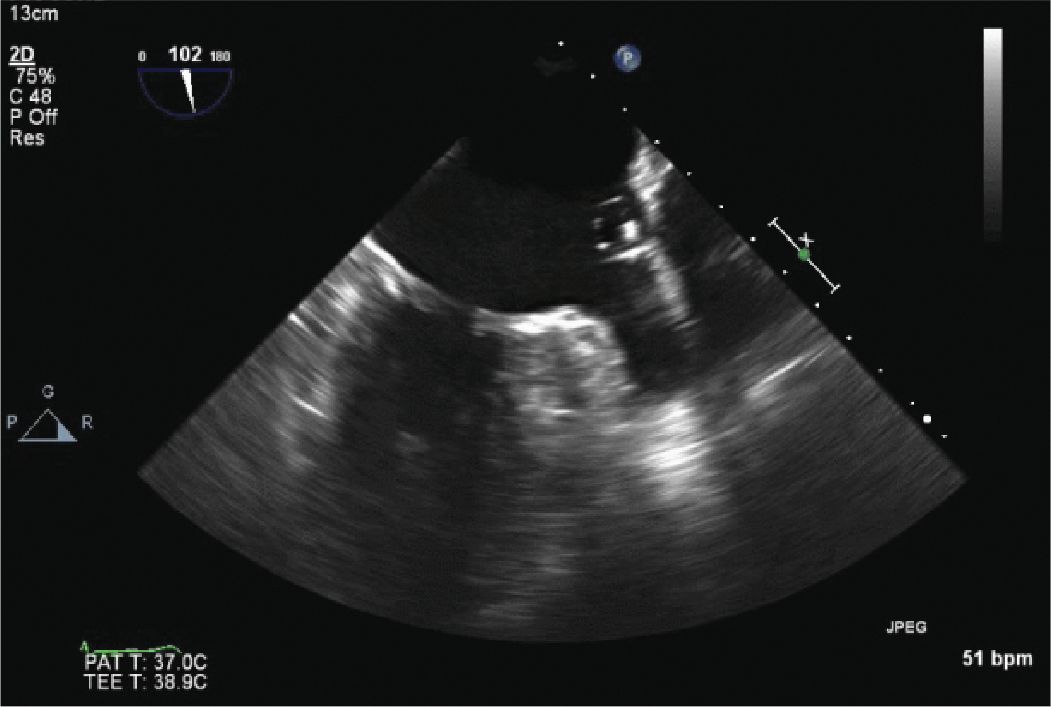
FIGURE 13-2-4 The catheter and balloon, used to help size the LAA and determine if the device will close the LAA, is seen in this image.
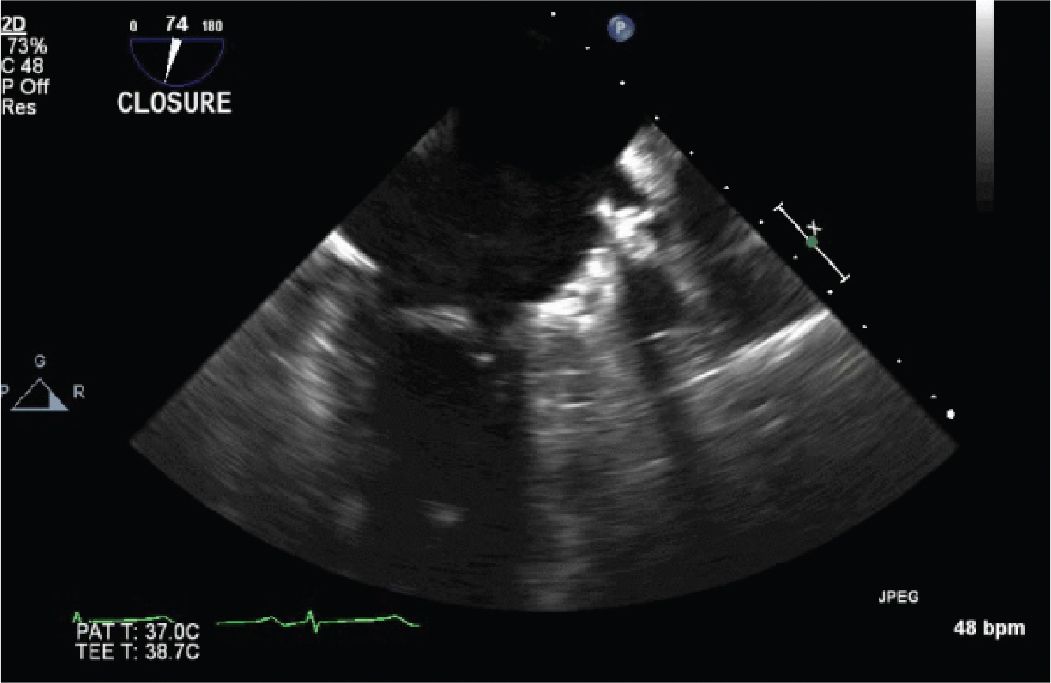
FIGURE 13-2-5 The LARIAT device has been placed, and the LAA is now closed from the LA.
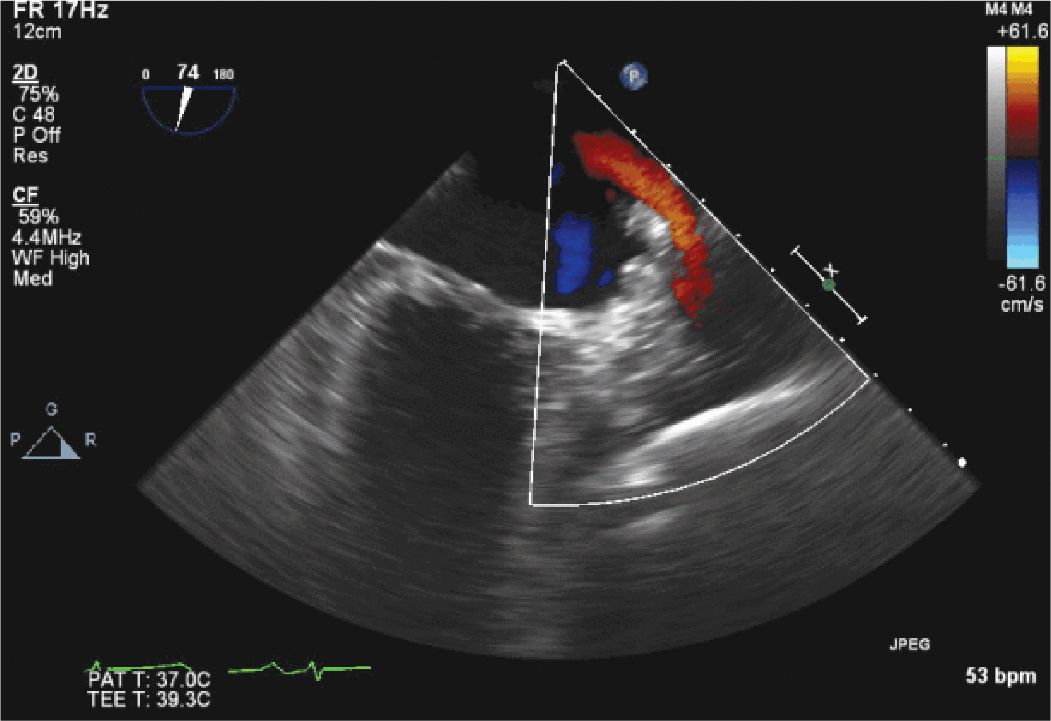
FIGURE 13-2-6 Color flow Doppler confirms that there is no flow from the LAA into the LA. The color flow (red jet) seen in the upper portion of the sector is normal flow from the left upper pulmonary vein.
CASE PRESENTATION
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


