13 Echocardiography in Cardiac Transplantation
Introduction
• Approximately 2200 heart transplantations are performed yearly in the United States, with a little over half of all transplantations performed for nonischemic causes of heart failure.
Assessing the Appropriateness of Heart Transplantation in Acute and Chronic Heart Failure Patients
• Establishing the suitability of heart failure patients for heart transplantation is a multidisciplinary process incorporating a variety of medical and psychosocial factors.
• In general, patients eligible for heart transplantation are less than 65 years old and have systolic or diastolic heart failure with New York Heart Association (NYHA) functional class IIIb or IV symptoms despite optimal medical therapy.
• Exclusion criteria for cardiac transplantation include cancer within the past 5 years, significant obesity, irreversible pulmonary hypertension, and other organ dysfunction not attributable to cardiac dysfunction.
• Echocardiography is an essential test used to evaluate potential heart transplant recipients and may provide information with regard to the etiology of heart failure and severity of the cardiomyopathy (ejection fraction in systolic heart failure, diastolic parameters in restrictive cardiomyopathies).
• Transthoracic echocardiography can provide clues to other abnormalities that should be addressed prior to consideration for transplantation, such as severe valvular abnormalities or constriction.
Evaluating Suitability of the Donor Heart
• Initial extracardiac factors, beyond those which exclude organ donation in general, may exclude the cardiac donor.
• These include advanced age (typically >55 years), prolonged ischemic time, or inappropriate donor size.
• Male donors over 70 kg are usually suitable in most cases. Body mass index and height are more accurately used for matching heart size to body weight in small donors.
• Echocardiography is now the imaging modality of choice in the evaluation of a potential cardiac donor, and special attention should be given to the echocardiographic factors noted below.
Step-By-Step Approach
Step 1: Assessment of Left Ventricular Hypertrophy
Key Points
• Anything more than mild left ventricular hypertrophy (LVH) in the donor heart typically precludes transplantation.
• It is important to obtain a true determination of LVH using both wall thickness criteria and, if possible, LV mass criteria. However, with poor acoustic windows, this may not always be feasible.
Step 2: Assessment of Valvular and Congenital Abnormalities
Step 3: Careful Assessment of Ejection Fraction, and Dobutamine Challenge if Indicated
The Transplanted Heart in the Postoperative Period
Step-By-Step Approach
Step 1: Assessment of Atrial Structure and Function
Key Points
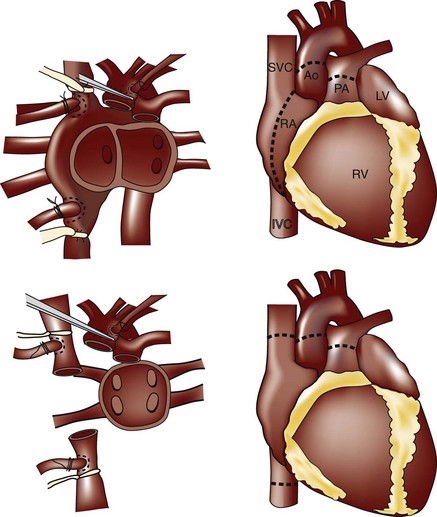
• Two major surgical approaches are used in heart transplantation that impact atrial structure and function: (1) the bi-atrial anastomosis (developed by Lower and Shumway), where recipient right and left atrial cuff tissue is left behind and sutured to the donor atria; and (2) the bicaval anastomosis, where a direct anastomosis is made to the venae cavae and a small portion of the recipient left atrium containing the pulmonary veins (Figure 13-1).
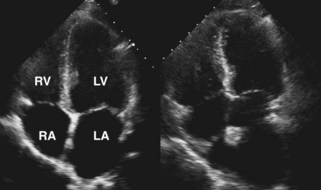
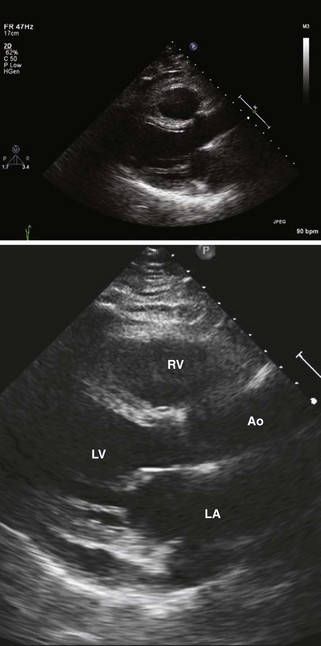
• In both cases, the left atrium is a composite of donor and recipient tissue and may look enlarged, especially as seen in the apical 4-chamber view (Figure 13-2).
• A dense ridge in the left atrium can be seen at the surgical anastomosis site and may be mistaken for a mass or thrombus, especially if a large cuff is present when there is donor and recipient size mismatch. This ridge otherwise allows discrimination between donor and recipient atrial tissue.
• The bi-atrial technique is associated with an increased risk of atrial thrombus, abnormalities in atrial filling, and poor LV hemodynamics. Hence the bicaval approach is now the technique of choice.
• Studies have shown that the bicaval anastomosis is associated with improved atrial geometry, decreased valvular insufficiency, decreased risk of arrhythmias, and decreased hospital stay.
Step 2: Assessment of Right Ventricular Dysfunction
Key Points
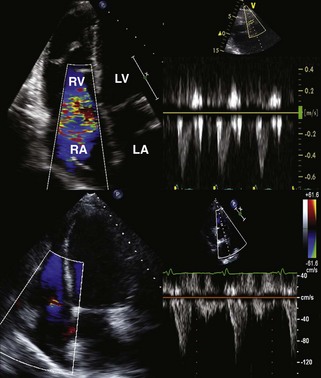
• Right ventricular dilatation and dysfunction are common immediately post-transplantation. The cause is multifactorial.
• In part this is related to chronic pulmonary venous hypertension and mild pulmonary vascular disease that may occur pre-transplantation in patients with chronic heart failure. There may also be associated ischemia and reperfusion injury during the process of transplantation.
• RV dysfunction is estimated to account for up to 50% of all cardiac transplantation complications and 20% of causes of early death after transplantation, as the donor ventricle cannot accommodate the acute increase in workload.
• The majority of cases of RV dilatation and dysfunction are treated medically and begin to improve over the course of 1 week post-transplantation.
• In the case shown in Figure 13-3, the marked RV dysfunction seen immediately postoperatively, with associated tricuspid regurgitation (TR), resolved with recovery to completely normal RV function and minimal TR within 1 month.
• Cases in which there is persistent RV dysfunction post-transplantation are associated with increased mortality.
Step 3: Assessment for Presence and Mechanism of TR
Key Points
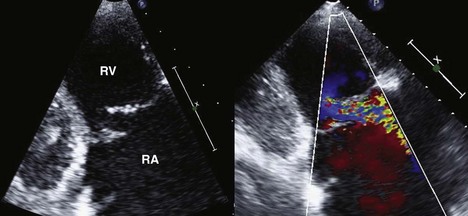
• Significant TR is the most common valvular abnormality after orthotopic heart transplantation (OHT). Significant regurgitation of the mitral and aortic valves, in contrast, is rare in the donor heart post-transplantation, unless there is acute rejection or prolonged ischemic time.
• Causes of TR early after transplantation include pulmonary hypertension, elevated pulmonary vascular resistance, and atrial dysfunction (more commonly seen with bi-atrial anastomosis). Typically TR improves with time as the pulmonary artery pressures normalize, usually over the first month after transplantation.
• Causes of TR that occur later include injury to the tricuspid valve and chordal apparatus by repeated endomyocardial biopsies, as well as allograft rejection. The TR from biopsy-related injury is usually more eccentric, and flail tricuspid valve leaflets may be seen (Figure 13-4).
• Bicaval anastomoses result in less TR, suggesting preservation of atrial structure and function and of tricuspid valve geometry are important in preventing TR.
Step 4: Assessment for Presence and Etiology of Pericardial Effusions
Key Points
• Pericardial effusions are common immediately post-transplantation, especially in patients with significant mismatch in size between donor and recipient.
• The differential diagnosis for an effusion postoperatively, however, includes acute allograft rejection and even purulent pericarditis, as these patients are immuno-compromised.
• Most postoperative effusions resolve within 1 month after transplantation without specific intervention.
• An enlarging pericardial effusion more than 1 month post-transplantation should raise concern for rejection or other causes, such as infection. Constriction is a rare complication post-transplantation, but has been reported.
Step 5: Assessment of Left Ventricular Function and Mass
Key Points
• LV systolic function should be preserved immediately postoperatively unless there is prolonged ischemic time for the donor heart or there is acute allograft rejection.
• Frequently, transplant recipients are placed on a combination of inotropic drugs such that hyperdynamic function may occur.
• The cause of LVH post-transplant is multifactorial, involving: post-transplantation hypertension, repeat episodes of rejection, effect of the immunosuppression regimen (especially calcineurin inhibitors), effects of chronic tachycardia, or injury and remodeling of the donor heart at the time of transplantation.
Step 6: Assessment for Possible Technical Complications of Surgery
Key Points
• Echocardiography in the transplant recipient is very useful to examine the sites of anastomosis, using color and spectral Doppler interrogation, in areas that may otherwise be overlooked in a routine echocardiogram of the native heart.
• In bicaval anastomosis, increased gradients across the inferior vena cava (IVC) and superior vena cava (SVC) should be assessed as these have adverse long-term hemodynamic consequences, including hepatic dysfunction.
• It is less common to encounter problems with the anastomosis of the great arteries, however, pulsed wave (PW) and continuous wave (CW) Doppler should be used to interrogate the pulmonary artery and aorta, especially in cases of donor-recipient size mismatch.
Echocardiographic Determinants of Cardiac Rejection
• However, profiling of inflammatory gene expression in combination with echocardiography may prove to be equivalent to routine scheduled biopsies in the diagnosis of rejection.
• Multiple echocardiographic techniques, including M-mode, two-dimensional (2D) echocardiography, conventional Doppler, and other indices of systolic and diastolic function, have correlated with episodes of biopsy-proven rejection.
• Unfortunately, no single echocardiographic technique to date has proved to have sufficient sensitivity and negative predictive value to be used as a sole predictor of acute rejection.
Step-By-Step Approach
Step 1: M-mode and 2D Echocardiography of the Left Ventricle
Key Points
• Acute allograft rejection is characterized by a cellular infiltrate in the myocardium, resulting in myocyte inflammation, edema, and ultimately contractile dysfunction.
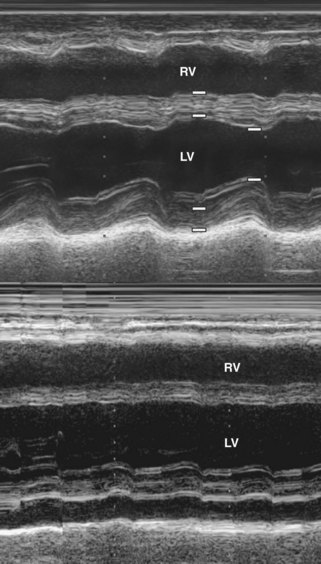
• M-mode of the left ventricle can detect the resulting increase in LV wall thickness, rise in LV mass, and decrease in LV fractional shortening and ejection fraction (Figure 13-5).
• 2D echocardiography complements M-mode findings but can also reveal an increase in myocardial echogenicity and the presence of an associated pericardial effusion (Figure 13-6).
• Unfortunately, neither M-mode nor 2D echo findings are specific for acute rejection; increases in wall thickness and LV mass can also occur as sequelae of hypertension, immuno-suppression, and chronic tachycardia post-transplantation.
• In the current era of potent immuno-suppression, acute rejection may present in a subtle fashion without frank symptoms or as a decline in LV systolic function until late in the course.
• Because decreases in M-mode and 2D measures of systolic function, such as fractional shortening and ejection fraction, may not occur until later in the presentation of acute rejection, these techniques are not sufficiently sensitive alone to exclude an episode of rejection.