Fig. 11.1
Pericardial adenosine deaminase activity in various causes of effusion: A box and whisker plot of the distribution of pericardial ADA activity in the various diagnostic classes. OTHER other pericardial effusions, INF infective, UNCERT pericardial effusions of unknown origin, CA malignancy (Reproduced by permission from Burgess et al. [24])
Interferon-γ (IFNγ, or type II interferon) is a cytokine that is critical for innate and adaptive immunity against viral and intracellular bacterial infections. IFNγ is an important activator of macrophages. The key association between interferon-γ and granulomas is that interferon-γ activates macrophages so that they become more powerful in killing intracellular organisms. Using a cutoff level for IFNγ of 200 pg/L as being diagnostic for tuberculous pericarditis resulted in a 100 % sensitivity and 100 % specificity for TB in the study of Burgess et al. [24]. In a subsequent publication this group found that a much lower level, IFN γ ≥50 pg/ml, concentration had 92 % sensitivity, 100 % specificity and a positive predictive value (PPV) of 100 % for the diagnosis of tuberculous pericarditis; in this study pericardial fluid ADA ≥40 U/l had 87 % sensitivity and 89 % specificity [26].
Improved specificity is found with the polymerase chain reaction (PCR) that detects the presence of tuberculous DNA in the effusion. Lee et al. found a sensitivity of 75 % and a specificity of 100 % [25]. Others have reported much lower sensitivity in documented tuberculous pericarditis [26].
Thus, in suspected tuberculosis acid-fast bacilli staining, mycobacterium culture or, adenosine deaminase (ADA), interferon γ (IFN γ), PCR analyses, pericardial lysozyme, or radiometric growth detection for tuberculosis can be performed on fluid depending on local availability of analyses. Diagnosis of neoplastic effusion can be made confidently with low levels of ADA and high levels of CEA. In addition, high ADA levels may predict the evolution towards pericardial constriction.
Complications of Pericardiocentesis and Drainage
Pericardiocentesis with echocardiography guidance is more often feasible when the effusion extends to the anterior pericardial space with >1 cm thickness of the effusion. Cases of loculated effusion or purely posterior effusion are best referred for surgery if drainage is necessary. In pericardial drainage appropriate case selection decreases complications. The most feared complication of 2-D echo guided catheter drainage is chamber perforation, which occurs infrequently, 16 patients (1.4 %) in the largest experience of 1,127 patients. Of these five (0.4 %) had lacerations that required surgery and one patient died postoperatively [5]. This complication may be avoided by careful selection of patients. Avoid patients without a clear target, a >1 cm anterior effusion under the needle. Moreover, the >1 cm clearance should be maintained throughout the cardiac cycle. Avoid patients with just posterior effusions. Monitor the ECG and if ventricular arrhythmias occur withdraw the needle. If perforation does occur and the catheter is placed in the right ventricle, the best course of action is to insert another catheter correctly into the pericardial space. Once its correct position is assured, withdraw the first catheter. Generally the right ventricular puncture will seal. If hemopericardium recurs after drainage at any time surgery should be done. Pneumothorax is also a possible complication of echo-guided pericardial drainage occurring in 1.1 % of patients. Of these five (0.4 %) of the patients required chest tube for lung re-expansion. This may be avoided by selecting an interspace that is directly over a large, clearly visualized, pericardial effusion, with minimal distance for the needle to traverse.
Another serious complication of pericardiocentesis is laceration or perforation of the coronary vessels. In addition, patients can experience air embolism, pneumothorax, arrhythmias (usually vasovagal bradycardia), and puncture of the peritoneal cavity or abdominal viscera. Internal mammary artery fistulas, acute pulmonary oedema, and purulent pericarditis are rarely reported.
Safety has been improved with echocardiographic or fluoroscopic guidance. Recent large echocardiographic series reported an incidence of major complications of 1–1.6 %. In contrast, in a large series of fluoroscopy-guided percutaneous pericardiocenteses, cardiac perforations occurred in <1 %, serious arrhythmias in 0.6 %, arterial bleeding in 1.1 %, pneumothorax in 0.6 %, infection in 0.3 %, and a major vagal reaction in 0.3 % [2, 27].
Additional Intrapericardial Therapy for Malignant Effusions
Malignant pericardial effusion and tamponade may complicate breast cancer, lung cancer, lymphomas, Kaposi’s sarcoma and leukemias. For many of these patients life expectancy is measured in weeks not years. Returning these patients home as soon as possible is an important goal. Reducing symptoms and improving the quality of life are the primary goals of treatment. Additional treatment besides drainage should be selected based on prognosis, and success rates. Oncologists, radiotherapists, and palliative care physicians should be consulted. For long-term prevention of recurrences, extended catheter drainage for several days seems to allow the two pericardial surfaces to adhere, decreasing the likelihood of fluid re-accumulation. Local installation of chemotherapeutic agents into the pericardial space is a reasonable approach for patients with overall expected shortened survival. Systemic chemotherapy or radiation therapy should be started as soon as possible for sensitive tumors. Therapeutic approaches vary for different tumor types. Use of ‘pure’ sclerosing agents like tetracycline has been replaced by agents with both sclerosing and antineoplastic activity (bleomycin or thiotepa), effective in breast cancer, at least when associated with systemic chemotherapy. Local chemotherapy with platinum, mitoxantrone, and other agents may have a role for local control, but beginning or resuming systemic chemotherapy may be the most important goal. Surgical window pericardiectomy is appropriate for patients with recurrent symptomatic re-accumulation.
Technique [3, 6, 28]
The patient is placed in a shallow right anterior decubitus position by placing a pillow or wedge underneath the right shoulder. Two-dimensional echocardiography is performed, specifically searching for the area of the chest wall where the pericardial fluid is closest to the skin [3, 4] (Fig. 11.2a). Our preferred location is at the apex of the left ventricle. As pericardial fluid accumulates, the lung is pushed out of the way and the apex becomes completely occupied by the pericardial effusion (Fig. 11.2). An excellent ultrasound window offers assurance that one is not over the lung. This window often extends as far as the left anterior axillary line in very large effusions. The pericardial fluid is generally 2–3 cm below the skin in this location. Other sites of entry are possible, including the parasternal spaces and the traditional subxiphoid entry. The subxiphoid route always requires a longer needle track with associated discomfort and demands a more precise direction of needle angle. The parasternal spaces, while completely accessible, require one to avoid the internal thoracic artery [29]. We have generally required an effusion >1 cm in the AP direction. Attempting to place the needle in a smaller effusion risks hitting the heart.
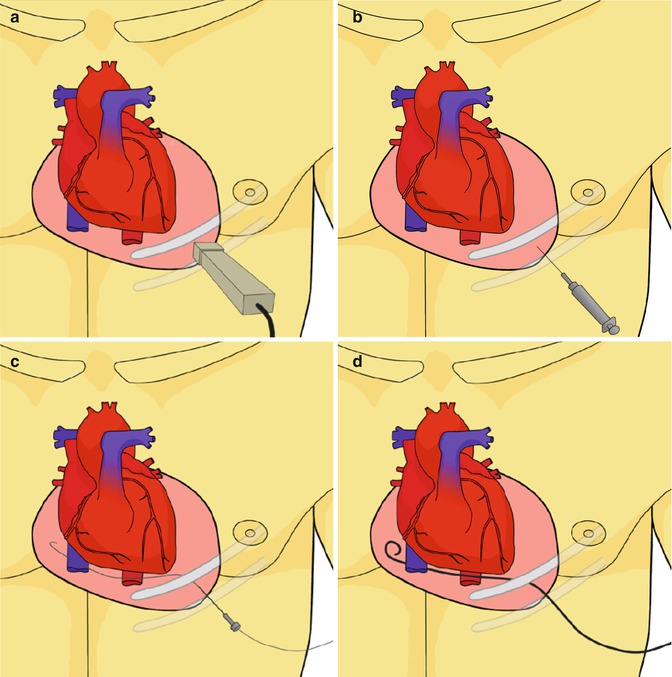

Fig. 11.2
The technique of echocardiographic guided pericardial drainage: (a) Using the 2D echocardiographic transducer the physician searches for the interspace location on the chest wall with the closest access to the large pericardial effusion. Generally the fluid will be 2–3 cm from the transducer. The transducer angle, cranio-caudad and medial-lateral from the center of the chest is noted. The interspace location is marked with a marker. The patient is prepped and draped. (b) Local anesthesia and low dose sedation is administered. Using the same position and angle noted above, the pericardial needle is gently advanced over the top of the rib into the pericardial space. (c) A J wire is advanced though the needle and into the pericardial space. If fluoroscopy is available the J wire is noted to course from the left side into the right without any intervening boundaries. This is a good confirmer that the wire is in the pericardial space and not in the heart. A sheath is placed over the wire, using standard Seldinger technique. Agitated saline may be injected at this point. (d) The pigtail catheter is then advanced into the pericardial space and evacuation of the fluid is begun (Reproduced with permission from Sherrid et al. [28])
The ideal interspace is chosen and a mark is made there with an indelible marker. With the transducer showing a “straight shot” into pericardial fluid one observes the transducer angle relative to the center of the chest: both medial-lateral and #2 cranio-caudad. Note also how far from the skin the fluid would be expected, and also the distance to the heart. Next, the ultrasound gel is cleaned off and the patient is prepped and draped.
The site of entry is just over the top of the rib. Local anesthesia is instilled, including both the skin and the intercostal muscle. The patient is lightly sedated. A ½ cm incision is made with a #14 scalpel blade.
The pericardiocentesis needle is gently inserted through the incision with the precise angle that had been indicated by the echo probe (Fig. 11.2b). The trocar is left in place until the needle is almost at the depth where fluid might be expected. It is then removed and gentle aspiration is applied using a 3 cc syringe. The pericardium is usually entered with a small pop. Once pericardial fluid is found, it is important to keep the needle still to avoid lacerating the heart. One advantage of the apical route is that the coronary arteries are small at the apex and there is less danger of lacerating a major coronary artery. Such a complication is more likely from the parasternal entry.
Bloody fluid is often aspirated in patients who have malignant effusions or post-surgical cases. However, on close inspection or after spinning, the fluid is serosanguineous not frank blood. Throughout the insertion of the needle and the sheath, a nurse observer watches the electrocardiogram; the occurrence of ventricular tachycardia, indicates that the heart has been hit and the needle should be withdrawn.
A guide wire with a small J is placed through the needle into the pericardial space (Fig. 11.2c). If the procedure has been done in the cardiac catheterization laboratory, typically the wire is seen to pass from the left chest into the right chest on fluoroscopy, as there are no intracardiac borders to confine it. This is a good marker of entry into the pericardial space. The needle is removed and then, using standard Seldinger technique, a sheath and introducer are inserted into the pericardial space and the guide wire and introducer are removed, leaving just the sheath.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


