8 Echocardiographic Tools for Cardiac Resynchronization Therapy
Background
• Cardiac resynchronization therapy (CRT) is a catheter-based therapy for patients with heart failure and evidence of left ventricular (LV) dyssynchrony.
• LV mechanical dyssynchrony is discoordinate (nonsimultaneous) ventricular contraction resulting from either an electrical timing delay or a functional abnormality.
• Atrial biventricular pacing addresses dyssynchrony at the atrio-, inter-, and intraventricular levels.
• There are two mechanisms of LV dyssynchrony:
• LV dyssynchrony resulting from abnormal LV contraction caused by diffuse tissue damage or nonuniform wall structure (e.g., scar tissue from a previous myocardial infarction [MI]) that interferes with normal LV activation, which may not respond to CRT.
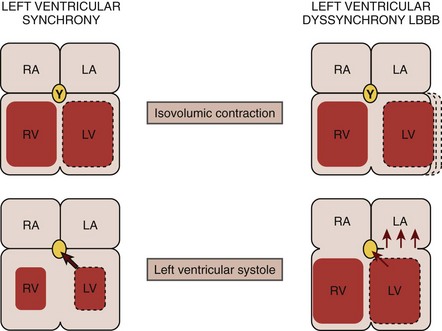
• In electromechanical dyssynchrony, infranodal conduction delay, most commonly in a left bundle branch pattern, results in a dyssynchronous LV contraction that is characterized by:
• Early systolic contraction of the “early activated” septum results in forces that are unopposed by the contralateral quiescent lateral free wall.
• The conversion of the septal forces into prestretch of the inactive lateral wall mitigates any effect on LV chamber pressure, delaying intracavitary pressure rise (maximum rate of change in pressure over time [dP/dt max]).
• Through slow intramyocardial conduction, the lateral wall is activated in late systole with generation of peak force that occurs in early diastole after aortic valve closure (AVC).
• Delayed contraction and pressure development of the “late activated” lateral wall is unopposed by the now relaxing septum, which leads to end-systolic stretching of this region.
• The relaxing septum creates an energy “sink” for the late developing lateral forces, resulting in a reduced stroke volume and thus cardiac output, with an increase in left ventricular end-systolic volume (LVESV) (Fig. 8-1).
• In addition, late activation of the postero-lateral papillary muscle results in suboptimal mitral valve closure and mitral regurgitation, further decreasing forward cardiac output.
Electrical Dyssynchrony
• Electrical dyssynchrony involves primary activation delay typically manifested by a widened QRS complex.
• While a prolonged QRS duration remains one of the criteria for the selection of patients for CRT, 30% to 40% of patients who fulfill this eligibility criteria do not show a symptomatic response or reverse remodeling.
M-mode and Doppler Approaches to Quantifying Mechanical Dyssynchrony
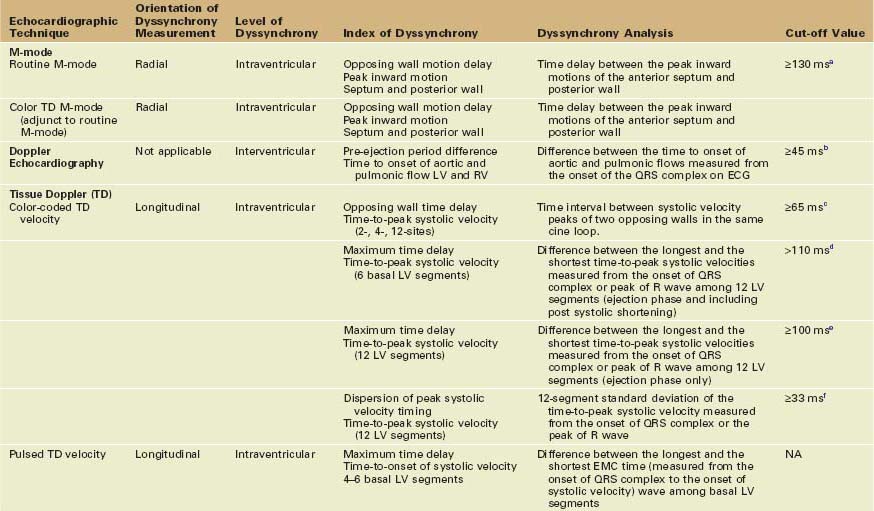
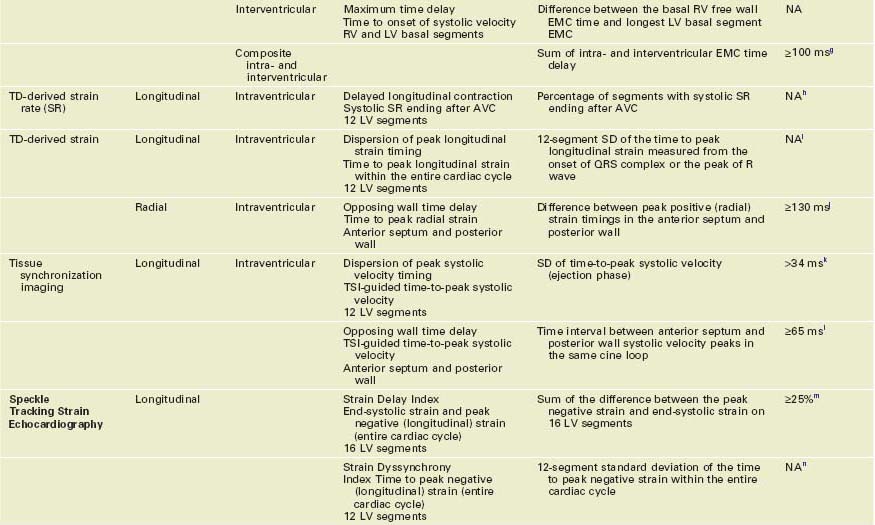
• A wide range of imaging modalities have been used to measure mechanical dyssynchrony, with echocardiography having accumulated the largest pool of evidence and being used more frequently in selecting patients for CRT (Table 8-1).
• Strengths and weaknesses of the various echocardiographic techniques used in the assessment of mechanical dyssynchrony are summarized in Table 8-2.
TABLE 8-2 STRENGTHS AND WEAKNESSES OF DIFFERENT ECHOCARDIOGRAPHIC APPROACHES FOR ASSESSING PATIENTS FOR CARDIAC RESYNCHRONIZATION THERAPY
| Echo Technique | Strengths | Weaknesses |
|---|---|---|
| M-mode | Simple and easy to measure Readily available on all echocardiographic systems Highly specialized training not required to perform analysis | Assesses only anterior septum and posterior wall Complex septal dynamics may result in difficulties in detecting peak inward motion Difficulty in detecting peak inward motion in cases of severe hypokinesis/akinesis in cases of infarction in the anterior septum or posterior wall Low prediction of responders/nonresponders |
| Color TD M-mode | A useful adjunct to M-mode determination of LV dyssynchrony Color changes in direction help identify the transition from inward to outward motion | Same as above |
| Doppler echocardiography | Readily available in all systems Requires no specialized training to perform analysis | Can only quantify interventricular dyssynchrony |
| Color-coded TDI | Can assess regional or segmental timing of myocardial contraction and relaxation A single view permits simultaneous comparison of the temporal profile of multiple segments by off-line analysis A highly comprehensive model of dyssynchrony can be provided by assessing the three apical views Useful in almost all patients | Requires dedicated hands-on training program Learning curve for offline dyssynchrony analysis Analysis in patients with atrial fibrillation is complex Presence of stationary artifacts or reverberations may alter the velocity plot and its postprocessing derivatives Largely affected by passive motion and tethering Malalignment of Doppler ROI increases as LV geometry becomes more globular Reproducibility of different echocardiographic indices has been challenged by PROSPECT trial results |
| Pulsed TDI | Available on most systems | Time consuming Susceptible to influences of breathing Requires manual transfer of the timing of the ejection interval Identification of peak velocity may be difficult Off-line analysis is not possible Affected by passive motion and tethering Prediction of response to CRT is less clearly studied |
| TDI-derived strain/strain rate/displacement | Theoretically translation and tethering independent (strain and strain rate) | Low signal-to-noise ratio and thus subject to error Angle dependent Can be influenced by reverberations and stationary artifacts Highly operator dependent |
| TDI-derived tissue synchronization imaging | Provides color-coded time-to-peak velocity data facilitating visualization of regional peak velocity timings Can be used to guide placement of ROI | Fundamental data are still TD velocity and therefore still require analysis of time-velocity curve for quantification of dyssynchrony |
| Speckle tracking echocardiography | Routine gray scale images are used Can be used to assess dyssynchrony in longitudinal, radial, or circumferential direction | Image quality dependent Frame rate sensitive Requires training and experience in performing analysis May be time consuming as analysis is performed using one cardiac cycle at a time New technique with limited clinical documentation Only one tomographic plane is assessed |
| RT3DE | Allows evaluation of dyssynchrony by analyzing LV wall motion in multiple apical planes during the same cardiac cycle. Better spatial resolution than a single plane | Reduced temporal resolution Online image acquisition and off-line analysis of RT3D echocardiographic data are technically demanding Requires training to perform analysis Vendor-specific cut-off value |
CRT, cardiac resynchronization therapy; ROI, region of interest; RT3DE, real-time three-dimensional echocardiography; TD, tissue Doppler; TDI, tissue Doppler imaging.
M-Mode Echocardiography
Acquisition
• Using a parasternal long axis or a short axis view, the M-mode cursor is positioned at the mid-ventricular level (papillary muscle level).
Data Analysis
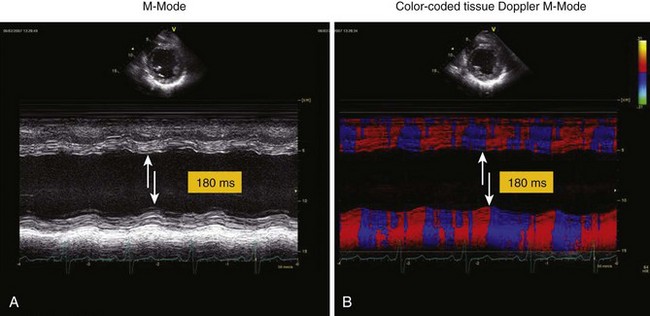
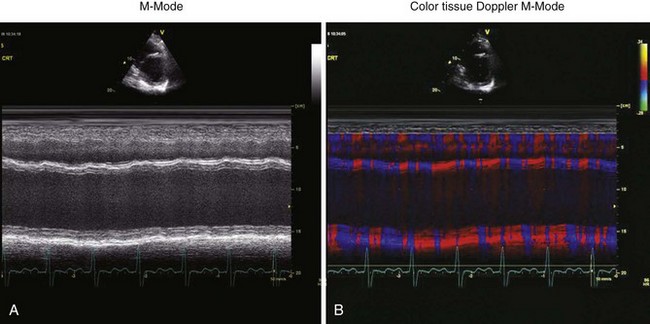
• The time delay between the peak inward motions of the anterior septum and posterior wall is identified and measured (Fig. 8-2).
• A septal-to-posterior wall motion delay (SPWMD) of at least 130 ms has been shown to predict LV reverse remodeling and clinical outcome after CRT.
Color Tissue Doppler M-mode
• Color-coded changes in direction may aid in identifying the transition from inward to outward motion in the septum and posterior wall.
Doppler Echocardiography
• Pulsed-wave Doppler flow velocities measured from the left ventricular outflow tract (LVOT) and the right ventricular outflow tract (RVOT) can provide information on both intra- and interventricular dyssynchrony.
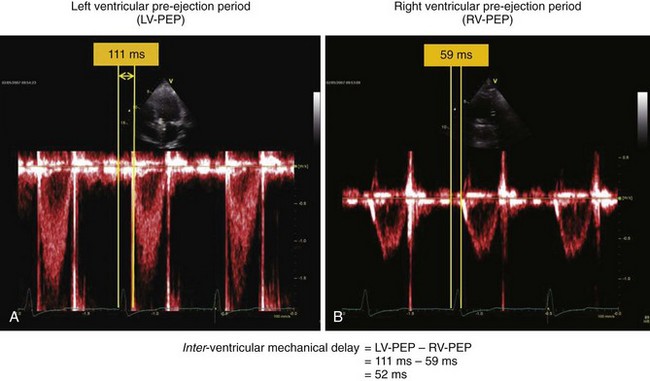
• LV pre-ejection time, measured from the onset of the QRS complex to the onset of LVOT flow, is prolonged in patients with LV dyssynchrony.
• Interventricular delay is defined as the difference between the right ventricular (RV) pre-ejection time, measured from the onset of the QRS complex to the onset of the RVOT flow, and the LV pre-ejection time (Fig. 8-4).
Tissue Doppler Echocardiography for Quantifying Mechanical Dyssynchrony
• The assessment of the longitudinal LV shortening velocities using TD from apical windows constitutes the largest body of literature in the quantification of dyssynchrony and is the principal method currently in clinical use.
• While both methods provide similar mechanical information, data obtained using each technique differ:
Color-coded Tissue Doppler
Acquisition
• To ensure maximal apical-to-near field left atrial imaging, two-dimensional (2D) imaging is optimized by adjusting the gain settings and time gain control settings for clear myocardial definition.
• Position the LV cavity in the center of the sector and aligned as vertically as possible to allow for the optimal Doppler angle of incidence with LV longitudinal motion.
• Activate color TD and adjust the sector to include the entire LV with the goal of achieving high frame rates (usually >90 frames per second [fps]).
• Capture three to five beats at end-expiration if the patient is able to suspend breathing transiently or at any phase where the image quality is optimal.
• Record in four standard imaging planes: apical four-chamber view, apical two-chamber view, apical long axis view, and parasternal short axis view at the level of the mid-LV.
Data Analysis
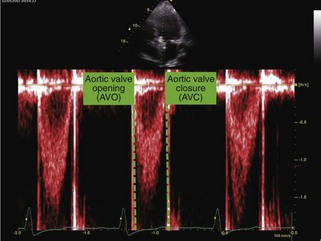
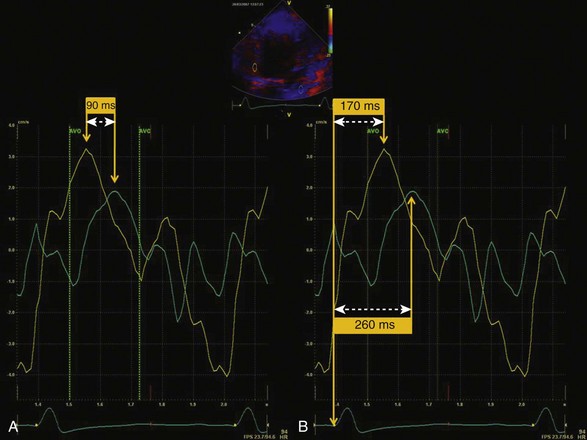
• Using the ECG as a time marker, LV ejection timing is determined from the beginning (aortic valve opening [AVO]) to the end (AVC) of pulsed Doppler flow of the LVOT (Fig. 8-5).
• Timing for AVO to AVC is superimposed as the ejection interval on the subsequent time-velocity curve analysis.
• Place the region of interest (ROI), minimum of 5 × 10 mm to 7 × 15 mm, in the basal (~1 cm above the mitral annulus) and mid-regions of opposing LV walls to determine time-velocity plots (four segments for each view).
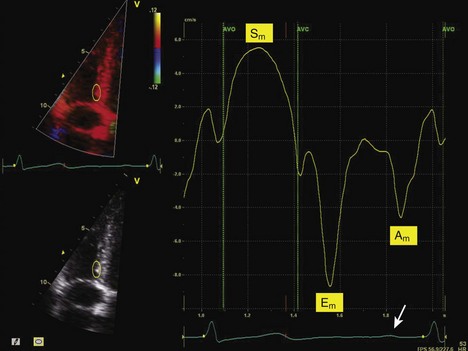
• Components of the velocity curve should be identified to check for physiologic signal quality (Fig. 8-6).
• Systolic velocity (S wave) is represented by a positive deflection within the ejection period as the myocardium moves toward the transducer during systole.
• Early diastolic velocity (E wave) is represented by a negative deflection after AVC as the myocardium moves away from the transducer during passive LV filling.
• Identify the site where the peak velocity during ejection is most reproducible by manually adjusting the ROI within the segment horizontally and longitudinally within the LV wall.
• In cases where the pre-ejection interval is severely prolonged, a very early peak may occur before the onset of LV ejection.
• Measure the time-to-peak systolic velocity relative to the onset of the QRS complex (or the peak of the R wave) for each segment for a total of 12 segments.
• Alternatively, the time delay between opposing walls can be determined by measuring the time interval from the peak S wave of one wall to the peak S wave of the opposing wall in the same cine loop.
Color-coded Tissue Doppler Approaches to Quantify Mechanical Dyssynchrony
• Opposing wall time delay technique:
• The simplest method to quantify LV dyssynchrony uses the basal segments of the apical four-chamber view to determine the time delay between the septum and the lateral wall (septal-to-lateral delay) (Fig. 8-7A).
• The four-segment model uses basal segments of the septum and the lateral, inferior, and anterior walls to determine the time delay between two opposing walls.
• The 12-segment model uses basal and mid-LV segments of the three standard apical views. The maximum difference in the time-to-peak velocity values among the four segments in each of the three apical views is determined as the maximal opposing wall delay.
• Yu Index or the mechanical dyssynchrony index:
• This index calculates the standard deviation (SD) of the time-to-peak systolic velocity in the ejection phase in the 12 basal and mid-LV segments (Fig. 8-7B).