12 Echocardiographic Evaluation of Ventricular Support Devices
Background
• Ventricular assist devices (VADs) were initially used to stabilize patients with acute cardiogenic shock (particularly post–cardiac surgery).
• Subsequently, they have been used as a “bridge to transplant,” “bridge to recovery,” or “bridge to decision” (stabilizing patients during work-up to decide transplantation candidacy) and as “destination therapy” (lifelong use).
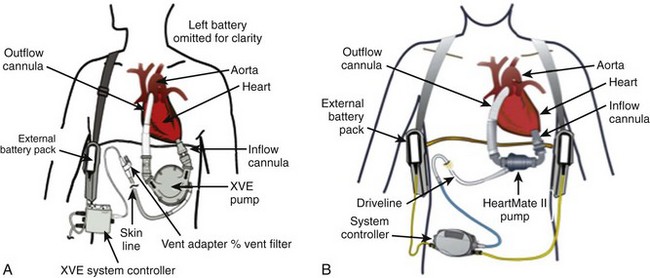
• Early-generation left ventricular assist devices (LVADs) mimic the pulsatile action of the heart and have inlet and outlet valves (Figure 12-1A; Table 12-1).
• Automatic mode on pulsatile pumps varies heart rate to maintain constant volume; therefore, pumping is asynchronous with the electrocardiogram (ECG).
• More recent LVADs are continuous flow, involving axial (rotating propeller) pumps, and have no valves (making them more durable) (see Figure 12-1B; see Table 12-1).
• In continuous-flow LVADs, rotor speed (in rotations per minute, rpm) and pressure difference between inflow chamber and outflow chamber (usually left ventricle and aorta, respectively) determine flow.
• Even in continuous-flow LVADs, pulsatility is present as a result of residual ventricular function and the fact that LVAD flow is higher when the aorta-LV pressure differential is low (systole) and lower when the differential is higher (diastole).
• Third-generation VADs are magnetically or hydrodynamically levitated, continuous-flow centrifugal (rotating plate) pumps with no bearings or valves, theoretically making them even more durable than second-generation VADs (see Table 12-1).
• The dysfunctional RV can respond well to LVAD implantation because of reduced afterload from lower pulmonary pressures.
• Alternatively, the RV function may worsen because of high preload from increased venous return secondary to increased left-sided output.
• Some patients receiving LVADs will require RV mechanical support, either temporarily (separate right ventricular assist device [RVAD]) or permanently (biventricular assist device [BiVAD]).
• Other patients with RV dysfunction can be supported medically with pulmonary vasodilators and inotropes.
• RVADs generally involve an inflow cannula placed in the right atrium and an outflow cannula placed in the proximal pulmonary artery.
• The total artificial heart is an alternative to VADs as a bridge to transplantation and consists of right and left pumping chambers attached to the native atria.
• The intra-aortic balloon pump (IABP) reduces systolic afterload on the left ventricle and can improve diastolic coronary flow via counterpulsation in the descending aorta.
• Percutaneous ventricular assist devices (PVADs) involve cannulae inserted via peripheral arteries and veins.
• PVADs can provide 2 to 6 L/min of cardiac output support and can be placed quickly in acute cardiogenic shock from (usually left but also right) ventricular decompensation and/or acute severe mitral regurgitation.
• PVADs are used primarily as a bridge to recovery and “bridge to bridge” (acute stabilization with subsequent implantation of a standard LVAD).
• PVADs are also used in high-risk percutaneous coronary interventions in patients with severely reduced LV ejection fraction (LVEF), and in percutaneous aortic valve procedures.
• PVADs provide better hemodynamic support than IABPs, but studies have not demonstrated improvements in mortality.
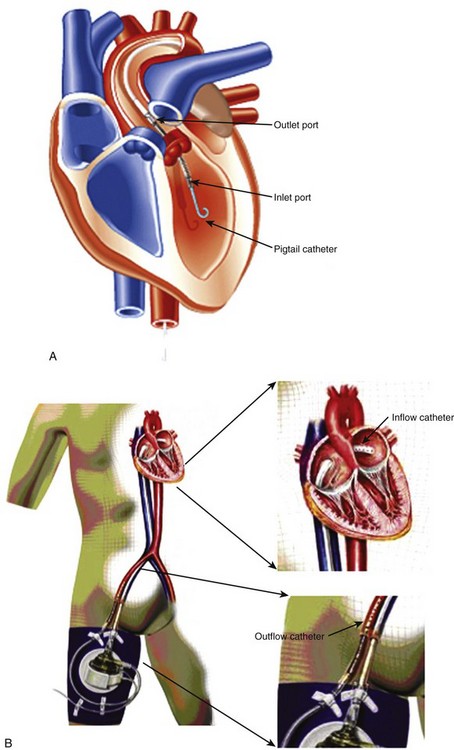
• The Impella Recover (Abiomed, Danvers, MA) can support the right or left ventricle and consists of a catheter in an inflow port and an outflow port that is advanced retrograde through the aorta or pulmonary artery into the ventricle (Figure 12-2A).
• The inflow port placed below the aortic or pulmonic valve draws blood from the ventricle and ejects it from the outflow port positioned above the valve.
• The TandemHeart (Cardiac Assist, Pittsburgh, PA) involves an inflow cannula placed through the femoral vein in the RA, across an interatrial septotomy, and into the left atrium. The outflow cannula is placed through the femoral artery into the abdominal aorta (see Figure 12-2B).
• Extracorporeal membrane oxygenation (ECMO) provides temporary cardiopulmonary support for patients with reversible cardiogenic shock and/or severe pulmonary failure.
• ECMO systems include an inflow cannula placed into the right atrium via the femoral vein or right internal jugular vein, and an outflow cannula in the femoral artery or ascending aorta.
TABLE 12-1 EXAMPLES OF VAD GENERATIONS
| Examples | Type |
|---|---|
| First Generation | |
| HeartMate XVE (Thoratec Corp.) | Pulsatile |
| Novacor pumps (WorldHeart Corp.) | Pulsatile |
| Second Generation | |
| HeartMate II (Thoratec Corp.) | Axial Flow |
| Jarvik 2000 (Jarvik Heart Corp.) | Axial Flow |
| Micromed DeBakey (Micromed Cardiovascular Inc.) | Axial Flow |
| Third Generation | |
| HeartWare HVAD (HeartWare Inc.) | Centrifugal |
| Duraheart (Terumo Heart Inc.) | Centrifugal |
| Levacor (WorldHeart Corp.) | Centrifugal |
| Total Artificial Heart | |
| Abiocor (Abiomed Corp.) | |
| Cardiowest (Syncardia Inc.) |
Overview of Echocardiographic Approach (Table 12-2)
LVADs
Preimplantation Echocardiographic Assessment
TABLE 12-2 APPROACH TO ECHOCARDIOGRAPHY IN LVADS
| Preoperative |
| Intraoperative |
| Diagnosis of LVAD dysfunction |
| Diagnose Obstruction |
| Diagnose Underfilling |
| Diagnose Regurgitation |
| Detect Vegetations and Thrombi on Valves and Cannulae |
| LVAD Optimization |
| Percutaneous VADs |
Echocardiography-Guided LVAD Optimization
• Optimizing LVAD pumping rates, pumping volumes, or rotor speeds uses echocardiographic measurements to balance proper preload and adequate decompression with excessive unloading leading to “suck-down” events (obstruction of the inflow cannula by trabeculation, chamber walls, papillary muscles, or chordal structures in the setting of an underfilled chamber).
Percutaneous VADs, IABP, and ECMO
• Echocardiography is useful in preimplantation assessment, placement, and follow-up of two of the currently available PVADs, IABPs, and ECMO.
Preimplantation Echocardiographic Assessment
Anatomic Imaging
Acquisition
• Standard echocardiographic imaging techniques are used to evaluate cardiac anatomy, with particular attention to ventricles, the left atrium, the great vessels, and the interatrial and interventricular septae.
• In patients with limited echocardiographic windows, the use of microbubble contrast for LV opacification may be necessary to provide an accurate LVEF assessment.
• Transesophageal echocardiography (TEE) is necessary to exclude LA and LA appendage thrombus, especially before planned LA cannulation.
Analysis
• Standard measurement and interpretation is done, with careful measurements of ventricular size (LV end-diastolic diameter [LVEDD]) and function, and detection of thrombi, aortopathy, and shunts.
• Real-time three-dimensional (3D) echocardiography may provide more accurate and reproducible measurements of LV volumes and LVEF.
• Severely enlarged RVs (defined in single-center experiences as > 85-mm end-diastolic diameter, > 200-mL end-diastolic volume, > 177-mL end-systolic volume, or short-axis to long-axis ratio ≥0.6), severe qualitative systolic RV dysfunction, tricuspid annular planar systolic motion (<7.5 mm in one study), or moderate to severe tricuspid regurgitation (TR), may require RVAD support (Table 12-3).
TABLE 12-3 PREDICTORS OF THE NEED FOR RVAD
| Echocardiographic Finding | Measurement |
|---|---|
| RV enlargement | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|