9 Echocardiographic Assessment of Treatment for Systolic Congestive Heart Failure
Echocardiographic Assessment of Systolic HF
• Echocardiography is an appropriate diagnostic test to investigate symptoms and signs of suspected HF.1 Echocardiography is also appropriate for guiding therapeutic decisions in HF patients and evaluating changes in clinical status.1 In addition to diagnosis, results from an echocardiogram (i.e., left ventricular ejection fraction [LVEF]) can provide important prognostic information.2
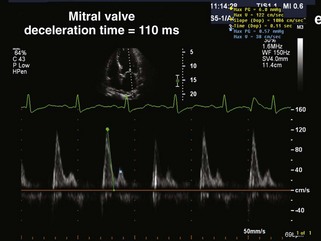
• In addition to LVEF, other echocardiographic parameters provide prognostic information. Specifically, in a study of cardiac mortality in HF patients with LVEF less than 40%, a restrictive transmitral flow pattern (Figure 9-1) by Doppler echocardiography was the best clinical predictor of cardiac death. Relative risk for cardiac death was estimated as 4.1 at 1 year and 8.6 at 2 years in the restrictive group compared with the nonrestrictive group.3 Several other markers of LV size and function (i.e., end-diastolic and end-systolic volume, myocardial performance index) and diastolic properties of the LV (i.e., pseudo-normal mitral inflow pattern) predict adverse prognosis in systolic HF.
• Three-dimensional (3D) echocardiography is a newer modality for measurement of LV volumes and LVEF. Studies have demonstrated that LV volumes measured by 3D echocardiography compare favorably with cardiac magnetic resonance imaging as a reference standard.4
• Novel echocardiographic measures of regional LV function, such as global longitudinal strain by speckle tracking echocardiography, may have superior prognostic value in HF patients over traditional measures of global function such as LVEF.5 Longitudinal and circumferential strain rates were independent predictors of outcome in myocardial infarction patients with LV dysfunction and/or HF.6 The effect of HF therapies on these novel echocardiographic measures represents an important area of ongoing investigation.
Medications
Beta Blockers
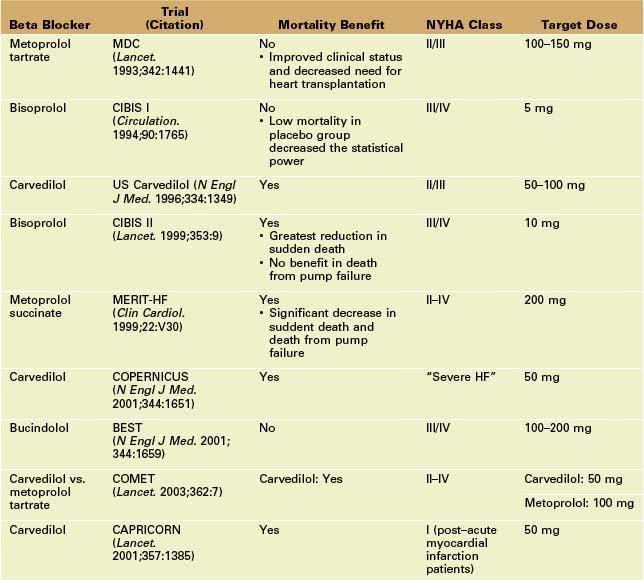
• Once thought to be detrimental, several beta-adrenergic blocking agents (beta blockers) are known to provide mortality benefits for systolic HF.
• Bisoprolol, carvedilol, and metoprolol are the beta blockers for which studies have documented mortality benefits in systolic HF.
• Improvement in LVEF with carvedilol in systolic HF results from decreased heart rate, improved chamber contractility, and afterload reduction.7
Key Points
• Large mortality trials of beta blockers in systolic HF are highlighted in Table 9-1. Beta blockers are essential first-line therapy in patients with HF and LV systolic dysfunction. Beta blockers are typically started at low doses and titrated to target doses.
• In the MDC trial, beta blockers produced favorable LV remodeling, with a larger increase in LVEF compared to placebo (13.0% vs. 6.0%, p < 0.0001). In general, treatment with beta blockers improves systolic function (increase in LVEF of 5% to 10%) and reduces symptoms.
Angiotensin-Converting Enzyme (ACE) Inhibitors
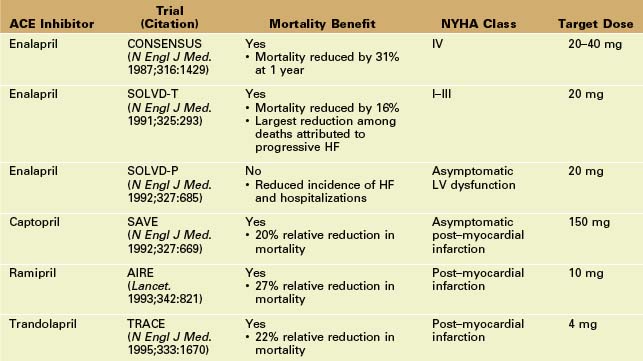
• Neurohormonal blockade of the renin-angiotensin-aldosterone system with ACE inhibitors has proven mortality benefit in systolic HF.
• Although ACE inhibitors have vasodilator properties, the main mechanism of benefit in HF patients is the prevention of angiotensin II–mediated LV remodeling. ACE inhibitors are first-line therapy for all patients with systolic HF.
Angiotensin II Receptor Blockers (ARBs)
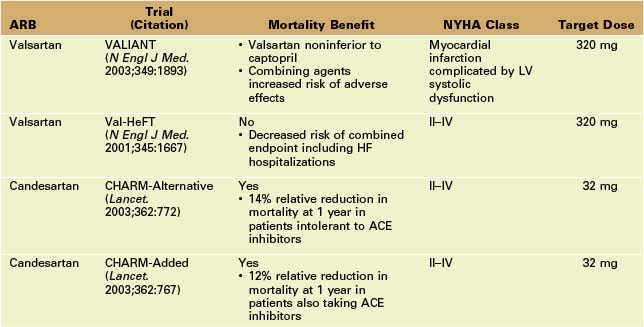
• ACE inhibitors do not completely inhibit renin-angiotensin activation, suggesting that alternative or additional strategies for inhibition (e.g., ARBs) may be useful. Since ARBs are generally more expensive, they are most commonly used as an alternative to ACE inhibitors in patients in whom a cough develops as the result of ACE inhibitor therapy.
Key Points
• ARBs can be used in addition to ACE inhibitors in patients with persistent symptoms despite an optimal dose of ACE inhibitor and beta-blocker. However, the risk of adverse effects (e.g., hyperkalemia, increase in serum creatinine, or hypotension) may be increased.
• The Valsartan Heart Failure Trial (Val-HeFT) echocardiographic substudy of 5010 patients with moderate HF demonstrated that valsartan therapy, taken with either an ACE inhibitor or a beta blocker, reversed LV remodeling (decrease in LV end-diastolic dimension and increase in LVEF).9
Aldosterone Antagonists
• Aldosterone antagonists can be used as an adjunct for further neurohormonal blockade in patients with systolic HF.
Key Points
• In the RALES trial, there was a 30% decrease in mortality when spironolactone was added to the therapeutic regimen for patients with New York Heart Association (NYHA) class III/IV HF and LVEF ≤ 35%. Spironolactone may exhibit this clinical benefit as a result of improvement in LV volumes and function.10
• In the EPHESUS trial, the addition of eplerenone to optimal medical therapy in patients with HF caused by acute myocardial infarction (LVEF ≤ 40%) resulted in a 15% decrease in mortality.11
• Recently, the addition of spironolactone in NYHA class I/II HF resulted in beneficial effects on LV remodeling and diastolic function. Specifically, in the spironolactone group, LVEF increased significantly from 35% to 39%, with a decrease in LV end-diastolic and end-systolic volumes and myocardial mass and an improvement in LV diastolic filling pattern.12
Hydralazine and Isosorbide Dinitrate
• In African American patients, the response to ACE inhibitors may not be as strong, and hydralazine and isosorbide dinitrate may have an expanded role in this patient group.
Implantable Cardioverter-Defibrillator (ICD)
• About one half of the deaths in patients with systolic HF are attributed to ventricular arrhythmias. An ICD reduces the risk of sudden cardiac death in patients with LV systolic dysfunction. The benefit of ICD therapy may not be apparent until 1 year or more after implantation of the device.
Cardiac Resynchronization Therapy (CRT)
• Intraventricular conduction delay (IVCD), identified by a 12-lead electrocardiogram QRS interval ≥ 120 ms, occurs in up to one third of patients with systolic HF. IVCD is associated with dyssynchronous LV contraction, leading to impaired emptying and possibly mitral regurgitation (MR).
• CRT may improve cardiac performance by increasing stroke volume and producing reverse remodeling, a process characterized by a reduction in LV volumes leading to improved systolic and diastolic function.15 CRT has also demonstrated a reduction in functional MR.16
• In randomized trials of severe systolic HF, CRT resulted in a reduction in symptoms and improved functional capacity, a reduction in the number of hospitalizations for worsening HF, and increased survival.17
• Current guidelines recommend CRT in patients with NYHA functional class III/IV HF, LVEF ≤ 35%, sinus rhythm, and a QRS duration ≥ 120 ms.14
Key Points
• In the MADIT-CRT trial,18 CRT in addition to an ICD in patients with NYHA functional class I/II, LVEF ≤ 30%, and QRS duration ≥ 130 ms resulted in improved LV function and reduced risk of worsening HF, compared to those who received an ICD alone. These effects were most pronounced in patients with a QRS complex ≥ 150 ms.
• In the RethinQ trial,19 CRT was not shown to increase the primary outcome measure of peak oxygen consumption in patients with NYHA class III symptoms and a narrow (< 120 ms) QRS interval and echocardiographic evidence of dyssynchrony (opposing wall delay > 65 ms; see below).
• Several echocardiographic parameters have been investigated as measures of mechanical dyssynchrony. These involve M-mode measurements as well as tissue Doppler imaging (TDI) for measurement of longitudinal tissue velocity or deformation (strain) of the myocardium. Several of the well-studied parameters of intraventricular dyssynchrony are described here:
• M-mode echocardiography: Septal-posterior wall motion delay (SPWMD), the time difference between peak inward motion of the ventricular septum and the posterior wall, can be obtained from parasternal short axis M-mode images (Figure 9-2A). An initial study showed SPWMD ≥ 130 ms predicted reduction in LV-end systolic volume index greater than 15% with a sensitivity of 100% and specificity of 63% in 20 patients after 1 month of CRT, and predicted improvement in LVEF greater than 5% and better prognosis at 6 months after CRT.20 However, this parameter was feasible in only one half of patients, and in follow-up reports SPWMD did not predict outcome after CRT.21