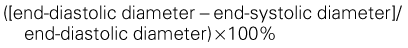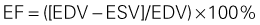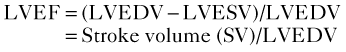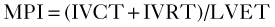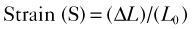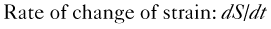6 Echocardiographic Assessment of Heart Failure Resulting from Coronary Artery Disease
Acute and Chronic Ischemic Heart Failure
Basic Principles
• Heart failure (HF) is caused by coronary artery disease (CAD) in more than 65% of patients and may be acute or chronic. There are two major causes of acute post–myocardial infarction (MI) HF:
• First, MI involving the loss of 25% to 35% of the LV precipitates acute HF because the remaining myocytes cannot sustain a normal cardiac output.
• Chronic HF results from progressive LV dilatation due to stretching of the infarct zone and expansion of the normal remote myocardium.
• LV dilatation increases wall stress that induces hypertrophy to normalize LV load and redistribute the elevated wall stress uniformly within the LV walls.
• Increased wall stress and neurohormonal activation drive the remodeling process, favoring LV dilatation and deterioration in function until a new equilibrium is reached between restraining forces exerted by the extracellular matrix and distending forces that promote LV dilatation.
• Myocardial repair involves collagen deposition and scar formation that provide an ideal substrate for re-entrant ventricular arrhythmias.
• Early diagnosis and treatment of HF is important because the prognosis of New York Heart Association (NYHA) class III/IV heart failure is poor (50% at 5 years).
• Doppler echocardiography provides a unique array of metrics for risk-stratifying patients with acute as well as chronic heart failure.
Key Points
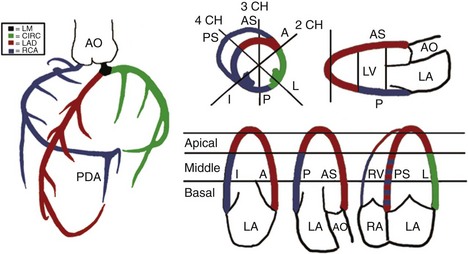
• Knowledge of the distribution of coronary artery blood flow in the 17-segment model of the LV is important for identifying the culprit coronary artery and the likely location of the flow-limiting coronary stenosis (Figure 6-2). Subtle RWMA at rest can be amplified by exercise or pharmacologic stress.
• In ischemic cardiomyopathy with a dilated LV and an ejection fraction (EF) less than 20%, it may be difficult to rule out RWMA because endocardial excursion is severely reduced.
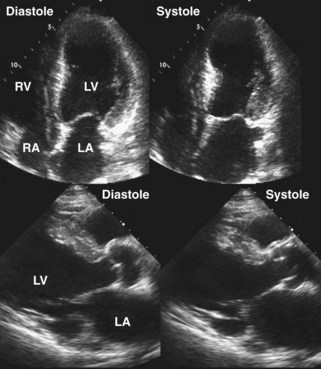
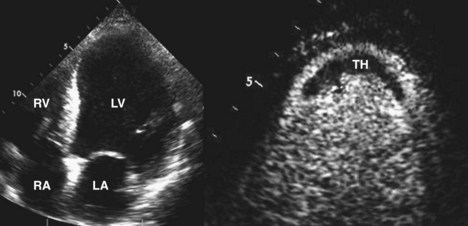
• Variation in the composition of the LV walls causes a heterogeneous acoustic signature from the myocardium, with increased brightness in infarcted regions due to the increased fibrosis (Figure 6-3).
• LV end-diastolic and end-systolic dimensions and volumes are both increased. The increase in end-systolic volume (ESV) is greater than the increase in end-diastolic volume (EDV), accounting for the fall in ejection fraction in acute HF.
• LV size as linear dimensions or LV volumes provides unique prognostic information for early risk stratification in HF. LV shortening and LVEF are both strong predictors of survival and adverse cardiovascular events.
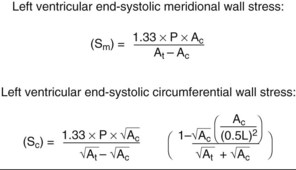
• Meridional and circumferential wall stress can be calculated from echocardiographic measurements of wall thickness, cavity radius, cavity length, and LV pressure (Figure 6-4). Wall stress is a major determinant of hypertrophy and structural and functional LV remodeling. Calculation of wall stress requires that two contingencies be met—that the LV behaves uniformly without regional abnormalities (RWMAs) and that the material properties of myocardium are constant. Neither of these contingencies is met in ischemic HF. However, longitudinal, radial, and circumferential myocardial strain can be measured and used as surrogates for wall stress.
• Early detection of complications of acute post-MI HF is essential. These include LV thrombus, MR, VSD, pericardial effusion, right ventricular (RV) infarction, increase in QRS duration, and LV dyssynchrony.
Quantification of LV Size and Function in HF
Basic Principles
Step 1
• M-mode echocardiography measurements of LV size in patients with CAD, RWMA, and abnormal LV shape may not be representative of the heart as a whole.
Step 2
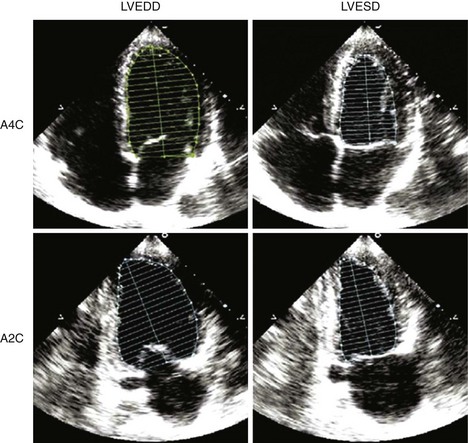
• Paired images of the apical 4-chamber and 2-chamber views should be used to estimate LV volumes using Simpson’s rule and indexed to body surface area (BSA) or height.
• LV cavity lengths should be similar in the two apical images to avoid foreshortening of the cavity, causing spuriously small volumes.
• Quantification of LV volumes at end-diastole and at end-systole (Figure 6-5) enables calculation of LVEF:
Step 3
• LVEF is often assessed visually by experienced echocardiographers, and correlates closely with LVEF calculated from digitized biplane images.
• The correlation above breaks down in large left ventricles with EF less than 30% and distorted LV cavity shape.
• The myocardial performance index (MPI) or Tei index has been used as an indicator of LV function that is determined as:
where IVRT is the isovolumic relaxation time, IVCT is the isovolumic contraction time, and LVET is the LV ejection time.
• Regional LV function is estimated visually using the 17-segment model during rest and stress. The overall wall motion score predicts cardiac outcome.
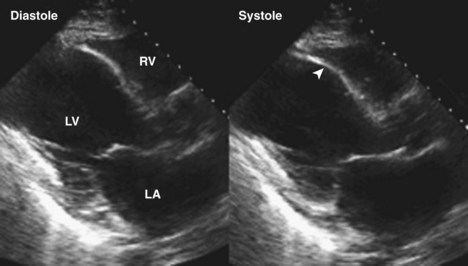
• Regional LV function can also be evaluated using tissue Doppler imaging (TDI), in which myocardial velocities are measured. In ischemic or infarcted regions, myocardial velocities are reduced to below normal (Figure 6-6).
Step 4
• Regional myocardial ischemia can be located by measuring regional strain using speckle tracking. Myocardial strain (S) is defined as the change in length (ΔL) at end systole as a function of resting length (L0) and strain rate is the rate of change of strain with time:
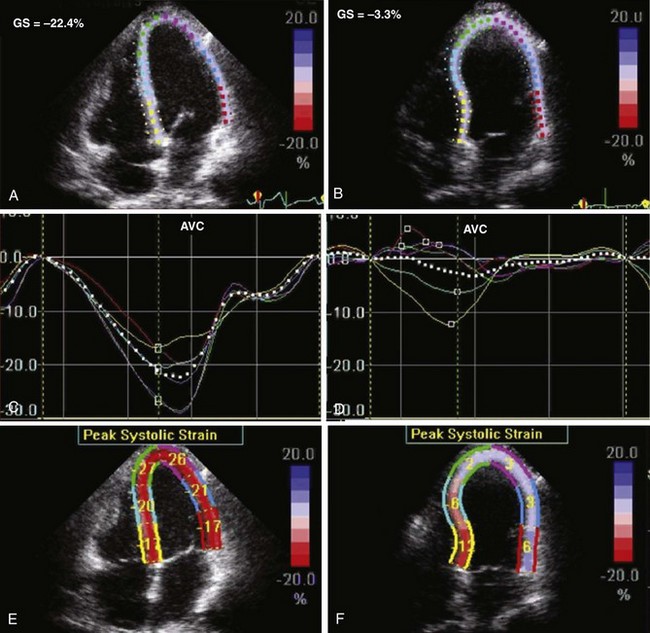
• Strain can be measured from the apex to base as longitudinal strain. The echo-derived values for myocardial strain have been validated against magnetic resonance imaging and have correlated closely both in healthy normal subjects and in a number of disease entities. Ischemic or infarcted myocardial segments in HF associated with CAD can be identified by the depressed regional and global longitudinal strain values and by the different times to peak strain from the various myocardial regions in the infarcted/ischemic versus normal segments (Figure 6-7).
Echocardiographic Detection of Thrombus in HF
Basic Principles
• Thrombus usually occurs at the LV apex or adjacent to a region of LV wall that is severely hypokinetic or dyskinetic. The amplitude of endocardial motion in the infarct zone is low, such that there is slow adjacent intracavitary blood flow velocity that looks like “smoke” and is a precursor of thrombus formation.
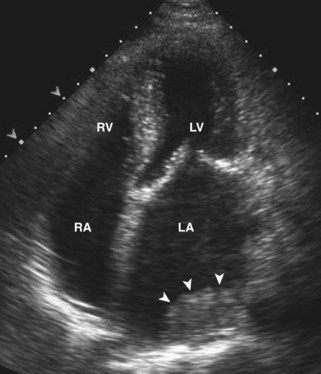
• Left atrial (LA) dilatation is common in HF due to high filling pressures. LA thrombus is especially frequent in the LA and the LA appendage in patients with atrial fibrillation and atrial flutter. Thrombus in the left heart chambers has the potential for cardioembolism, causing stroke or loss of organ function. Detection of LV thrombus is of paramount importance because appropriate anticoagulation can be instituted immediately.
Key Points
Step 1
• Thrombus appears as a homogeneous mass with no discernible architecture and a different acoustic signature from the myocardium. Thrombus is most frequently located at the LV apex, adherent to an infarct zone or in the LA (Figure 6-9).
Step 2
• The morphology of the thrombus determines its likelihood for embolization. The features of thrombus associated with embolism are large size, convexity toward the LV cavity, and excessive mobility.
• The LV apex should be examined in all three standard apical views as it may be difficult to differentiate thrombus from LV trabeculations at the apex. This may be facilitated by using a high-frequency short-focus transducer to acquire “off-axis” images, apical long axis and tomographic transaxial images to include the LV apex (see Figure 6-8).
Step 3
• If there is still doubt as to the presence of LV thrombus, enhancing endocardial definition with intravenous contrast presents the thrombus as a filling defect within the contrast-filled LV cavity (Figure 6-10). Transesophageal echocardiography can be used to confirm or rule out intracardiac thrombus.
Postinfarction Ventricular Septal Defect
Basic Principles
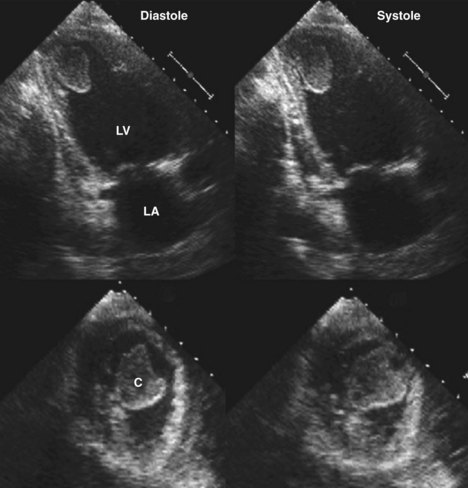
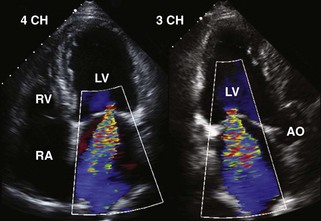
• Persistent refractory acute HF or new-onset intractable HF several days post-MI should suggest severe LV volume overload due to MR (Figure 6-11) or rupture of the interventricular septum into the right ventricle (VSD) (Figure 6-12).
• Distinguishing the two mechanical lesions purely on the auscultatory features of a harsh holosystolic murmur is unreliable despite claims to the contrary.
• Doppler echocardiography removes the clinical doubt because color flow Doppler technology enables unequivocal localization of the VSD and at the same time can rule out MR (see Figure 6-12).
• The size of the defect in the ventricular septum can often be visualized directly and semiquantified by the maximal width of the color flow Doppler jet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree