SECTION I
CLINICAL CASE PRESENTATION
An actively smoking 85-year-old man with a history of previous myocardial infarction, permanent atrial fibrillation, peripheral arterial disease, coronary artery bypass surgery 12 years prior, and an LV ejection fraction of 20% presented to an outside hospital with severe respiratory distress that required endotracheal intubation and mechanical ventilation. He denied having experienced chest discomfort. He was transferred to our institution for further care. On arrival, a 12-lead electrocardiogram (ECG) showed atrial fibrillation with right bundle branch block and lateral ST-segment depressions. Chest radiography revealed cardiomegaly and the presence of bilateral pleural effusions and interstitial edema. A brain natriuretic peptide (BNP) level was >4000 pg/mL; cardiac troponin I levels were within normal limits. Echocardiography demonstrated LV dilatation and evidence of regional wall motion abnormalities with akinesis and thinning in the distributions of the circumflex and right coronary arteries and severe hypokinesis in the distribution of the anterior descending artery. The left atrium was markedly dilated, and LV filling pressures were elevated. Moderate to severe mitral regurgitation (MR) was demonstrated and the pulmonary artery systolic pressure (PASP) was estimated to be about 50 to 55 mm Hg. With aggressive diuresis, the patient was able to be separated from the ventilator within 24 hours. Appropriate guideline-recommended medical therapy was initiated and titrated to tolerance; smoking cessation was stressed. A subsequent SPECT-thallium viability study confirmed the depressed LVEF and documented infarct (scar) involving the entirety of the distributions of the circumflex and right coronary arteries; no evident ischemia was demonstrated in the LAD distribution. After discussion with his primary cardiologist, and taking into consideration his clinical status, a decision not to pursue coronary angiography and prophylactic ICD implantation was made.
CLINICAL FEATURES
As demonstrated by this case vignette, a history of prior coronary heart disease (CHD) is evident in most patients, although some may offer no such history.
• Patients may present with an asymptomatic reduction in left ventricular ejection fraction (LVEF), but most present with symptoms and signs of heart failure, such as fluid retention, exertional dyspnea, fatigue, or exercise intolerance, with or without angina pectoris.
• A history of prior CHD with prior revascularization procedures or myocardial infarction, peripheral artery disease or significant risk factors for CHD is common and should prompt further investigation because in up to two-thirds of cases of heart failure with reduced LVEF the underlying cause is found to be ischemic heart disease.
• In a large retrospective database study of patients undergoing coronary angiography for LVEF ≤40%, three-quarters of the patients with ischemic cardiomyopathy had greater than or equal to 75% stenoses of the left anterior descending artery, and 46% had three-vessel CHD.1
• Patients with ischemic cardiomyopathy more often were older, men, and possessed a higher incidence of risk factors for CHD compared to patients with nonischemic cardiomyopathies. In this database, patients with nonischemic cardiomyopathies had lower LVEFs and longer duration of heart failure symptoms.
• It should be appreciated that as many as one-third of patients with nonischemic cardiomyopathy may complain of chest pain.1
EPIDEMIOLOGY
• Currently, it is estimated that 6 million people in the United States live with heart failure; approximately 40% to 50% have preserved LVEF. The remaining individuals have heart failure with impaired systolic function (systolic CHF).
• Coronary heart disease is the leading cause of systolic heart failure in industrialized nations. Among heart failure patients with reduced LVEF, it is estimated that 60% of cases occur secondary to an ischemic etiology.
• About 7% of heart failure patients with previously unexplained cardiomyopathies are subsequently identified to have ischemic heart disease as the cause of LV dysfunction.2 Among a cohort of patients with myocardial infarction and no previous history of heart failure, approximately 40% developed heart failure (most often with reduced LVEF) over a follow-up period averaging 6.6 years.3
• Compared to patients with nonischemic etiology, a lower 5-year survival is found among patients with ischemic cardiomyopathy.1
PATHOPHYSIOLOGY
Ischemic cardiomyopathy refers to significantly impaired LV systolic function (ejection fraction ≤35%-40%) resulting from the presence of underlying CHD.
• Impaired systolic function may be due to irreversible loss of myocardium (scarring) with resultant adverse ventricular remodeling, reduced function due to ischemic but still viable myocardium (hibernation and/or repetitive stunning), or a combination of the two. Multivessel CHD with involvement of the left anterior descending artery is common.1
• In the presence of extensive scar, revascularization would not be expected to lead to improved LVEF. Targeted revascularization would be expected to improve symptoms and prognosis in the presence of significant amounts of viable myocardium.
• Among patients with CHD, underlying risk factors such as systemic hypertension, diabetes mellitus, and/or LV hypertrophy may also contribute to the observed ventricular dysfunction.
ECHOCARDIOGRAPHY
For patients presenting with new onset heart failure symptoms, echocardiography has a Class I indication in the initial evaluation.4 Recently published appropriate use criteria would consider use of echocardiography as “appropriate” for this clinical indication.5 While the history, physical examination, and ECG often help identify heart failure patients with ischemic etiology, the echocardiographic picture may not always be quite so clear.
• It may be difficult to distinguish an ischemic from a nonischemic dilated cardiomyopathy on the basis of echocardiography alone. Findings common to both conditions include the presence of a dilated, hypocontractile LV cavity with evidence for reduced systolic and diastolic performance.
![]() Wall thickness may be normal or reduced.
Wall thickness may be normal or reduced.
![]() With advanced disease, a more spherical LV geometry may be seen in both conditions.
With advanced disease, a more spherical LV geometry may be seen in both conditions.
![]() Dilatation of the left atrium, right atrium, and/or right ventricle (RV) may be present. The RV may display reduced (systolic) performance due to an imposed afterload (such as increased PA systolic pressure or pulmonary vascular resistance) and/or pathological involvement.
Dilatation of the left atrium, right atrium, and/or right ventricle (RV) may be present. The RV may display reduced (systolic) performance due to an imposed afterload (such as increased PA systolic pressure or pulmonary vascular resistance) and/or pathological involvement.
![]() Intracavitary thrombus may be seen coincident with areas of akinesis (Figure 5-1-1). Similar to findings that may be encountered with nonischemic dilated cardiomyopathy, the M-mode examination may show an increased E-point-septal separation distance (indicative of reduced LV stroke volume), a B-bump (interrupted A-C closure) indicating elevated LV end diastolic (and LA) pressure, and gradual closure of the aortic valve echo during systole (Figures 5-1-2 and 5-1-3).
Intracavitary thrombus may be seen coincident with areas of akinesis (Figure 5-1-1). Similar to findings that may be encountered with nonischemic dilated cardiomyopathy, the M-mode examination may show an increased E-point-septal separation distance (indicative of reduced LV stroke volume), a B-bump (interrupted A-C closure) indicating elevated LV end diastolic (and LA) pressure, and gradual closure of the aortic valve echo during systole (Figures 5-1-2 and 5-1-3).
![]() Doppler assessment of diastolic function, including the E/A ratio, E/e’ ratio, deceleration time, and analysis of the pulmonary vein spectral profile, rarely is normal among patients with advanced ischemic or nonischemic cardiomyopathies.
Doppler assessment of diastolic function, including the E/A ratio, E/e’ ratio, deceleration time, and analysis of the pulmonary vein spectral profile, rarely is normal among patients with advanced ischemic or nonischemic cardiomyopathies.
![]() Increased pulmonary artery systolic pressures and mild or greater functional mitral regurgitation can be observed occur in the majority of patients.
Increased pulmonary artery systolic pressures and mild or greater functional mitral regurgitation can be observed occur in the majority of patients.
• The hallmark of ischemic heart disease is the presence of regional wall motion abnormality in the distribution of known coronary artery territories.
![]() Among patients with dilated cardiomyopathy, regional wall motion abnormalities must be interpreted with caution as regional variation in systolic function may be seen in nonischemic cardiomyopathies.
Among patients with dilated cardiomyopathy, regional wall motion abnormalities must be interpreted with caution as regional variation in systolic function may be seen in nonischemic cardiomyopathies.
![]() When a substantial area of scar (thinning [<6 mm wall thickness] with increased echo-reflectivity) (Figures 5-1-4 and 5-1-5) corresponding to the known distribution of a coronary artery territory is present or an LV aneurysm (apical or inferobasal) is observed, the likelihood of ischemic etiology is increased.
When a substantial area of scar (thinning [<6 mm wall thickness] with increased echo-reflectivity) (Figures 5-1-4 and 5-1-5) corresponding to the known distribution of a coronary artery territory is present or an LV aneurysm (apical or inferobasal) is observed, the likelihood of ischemic etiology is increased.
![]() Functional studies, such as dobutamine stress echocardiography, myocardial perfusion imaging, or positron emission tomographic imaging may be useful in making this distinction. In many instances, however, another imaging modality, such as cardiac magnetic resonance with gadolinium enhancement or coronary angiography (most commonly), will be required.
Functional studies, such as dobutamine stress echocardiography, myocardial perfusion imaging, or positron emission tomographic imaging may be useful in making this distinction. In many instances, however, another imaging modality, such as cardiac magnetic resonance with gadolinium enhancement or coronary angiography (most commonly), will be required.
• Deformation (strain/strain rate) imaging can provide an objective measure of both global and regional myocardial function and thus may offer additional information in distinguishing myocardial infarction from ischemic but viable tissue.
![]() Deformation imaging can help distinguish between passive motion (such as with tethering of infarcted tissue by adjacent segments) and active contraction characteristic of viable tissue and may be predictive of the transmural extent of infarction.
Deformation imaging can help distinguish between passive motion (such as with tethering of infarcted tissue by adjacent segments) and active contraction characteristic of viable tissue and may be predictive of the transmural extent of infarction.
![]() Postsystolic shortening has been reported to be a specific marker of ischemic tissue, and indexed post-systolic stain rates may help define transmural extent of infarction.6
Postsystolic shortening has been reported to be a specific marker of ischemic tissue, and indexed post-systolic stain rates may help define transmural extent of infarction.6
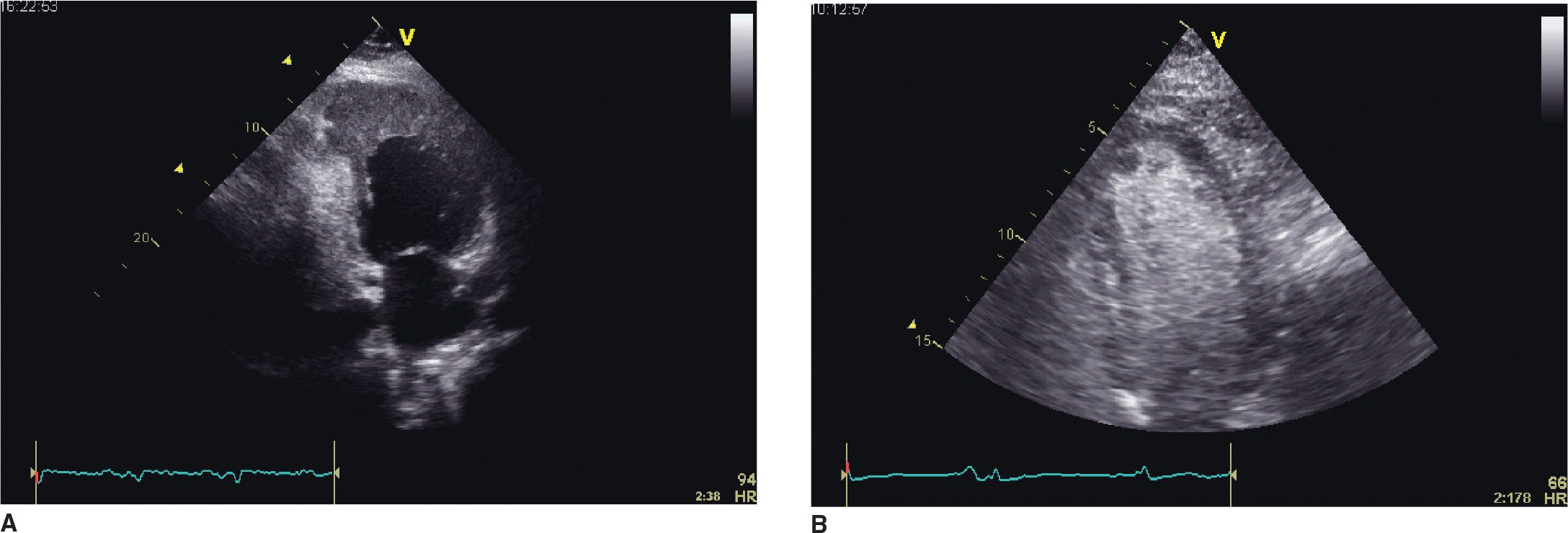
FIGURE 5-1-1 (A) Apical 2 chamber view recorded in a patient with prior myocardial infarction and a large left ventricular apical aneurysm. Echodensity consistent with thrombus fills the anuerysmal area. (B) Microbubble contrast-enhanced apical 4 chamber view showing a protruding, rounded filling defect adjacent to the apical portion of the septum. In the presence of a focal wall motion abnormality in this segment, thrombus is highly likely as the etiology.
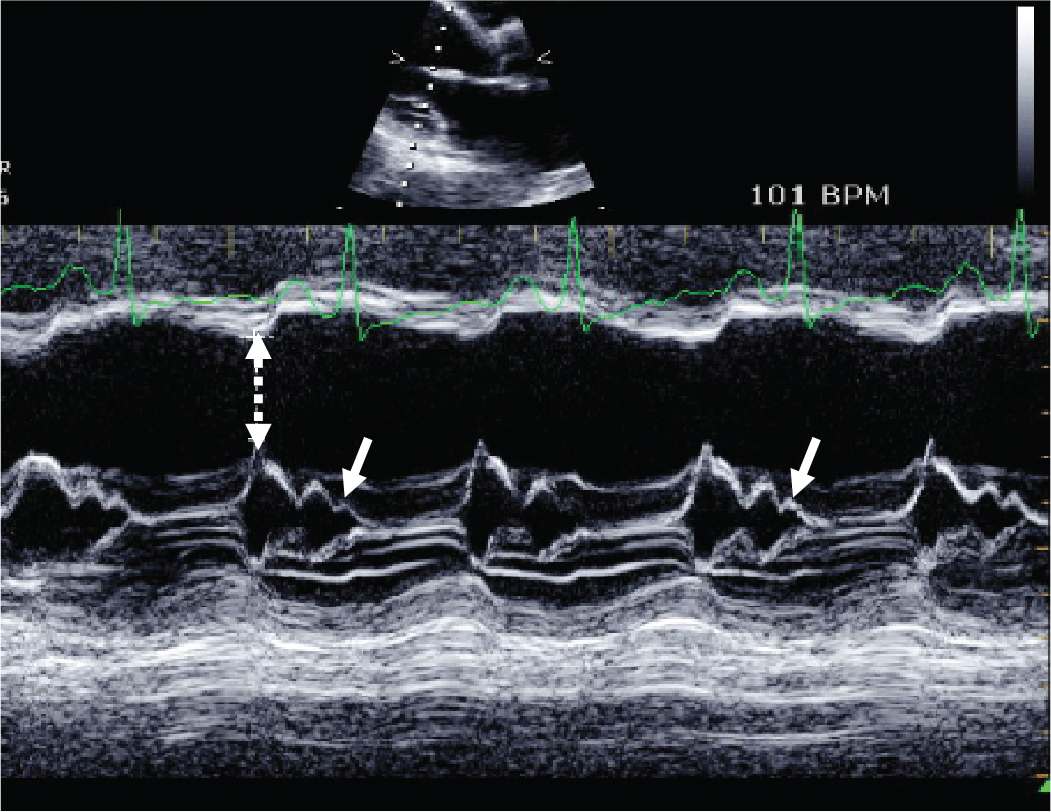
FIGURE 5-1-2 M-mode recording made through the mitral valve demonstrating increased E-point septal separation distance (stippled arrow) and interrupted A-C closure (B-bump) [solid arrows]. The presence of an increased E-point septal separation distance is indicative of reduced stroke volume while a B-bump is indicative of increased left ventricular end-diastolic pressure.
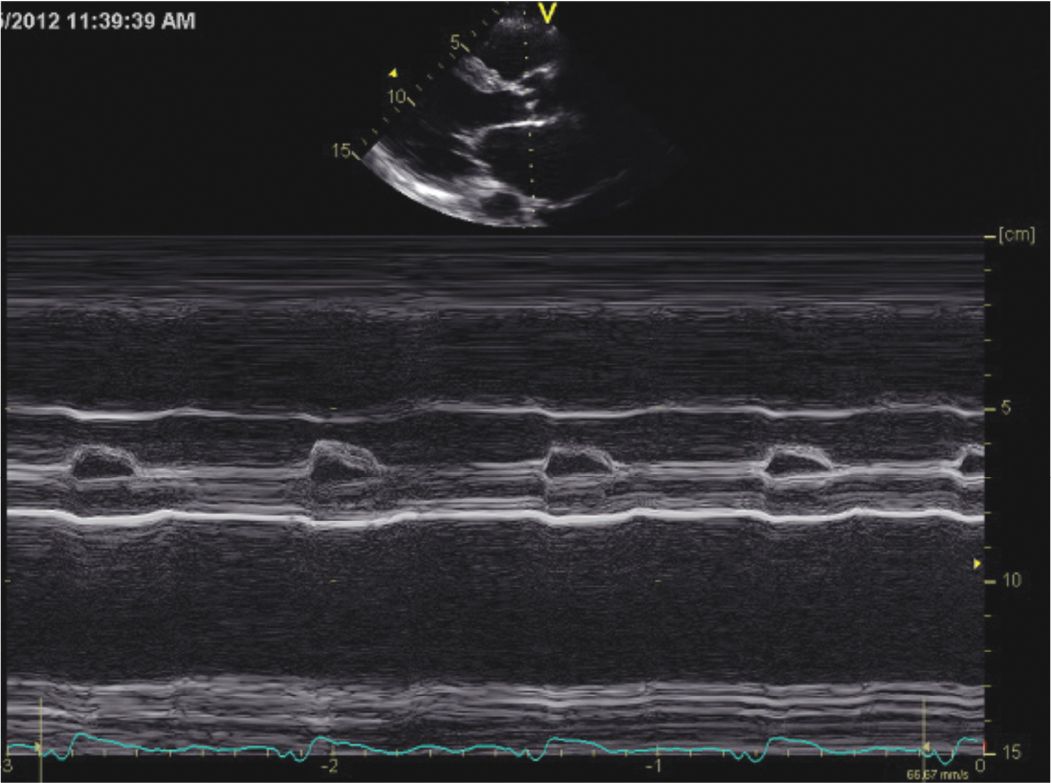
FIGURE 5-1-3 M-mode recording of the aortic valve in systole demonstrating that after initial opening the leaflets drift to closure gradually rather than exhibiting the usual “boxcar-like” configuration. This finding is indicative of reduced stroke volume.
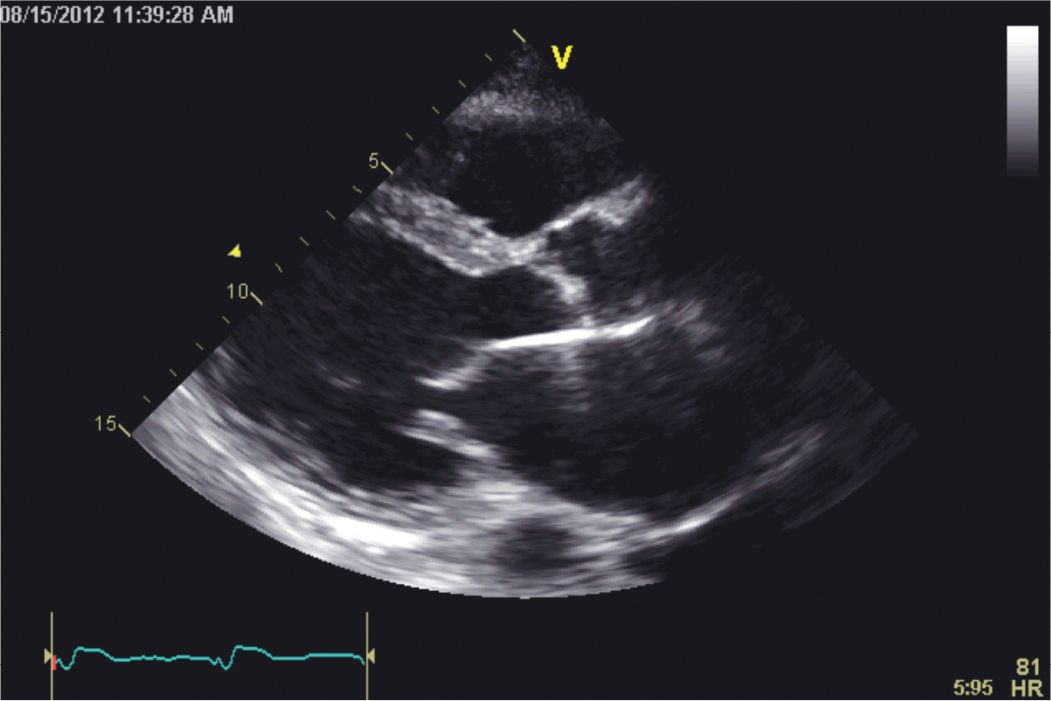
FIGURE 5-1-4 Parasternal long-axis image showing a dilated left ventricular cavity with thinning and akinesis of the visualized portion of the inferolateral wall. The interventricular septum is hypokinetic. The excursion of the mitral valve is reduced due to the low stroke volume.
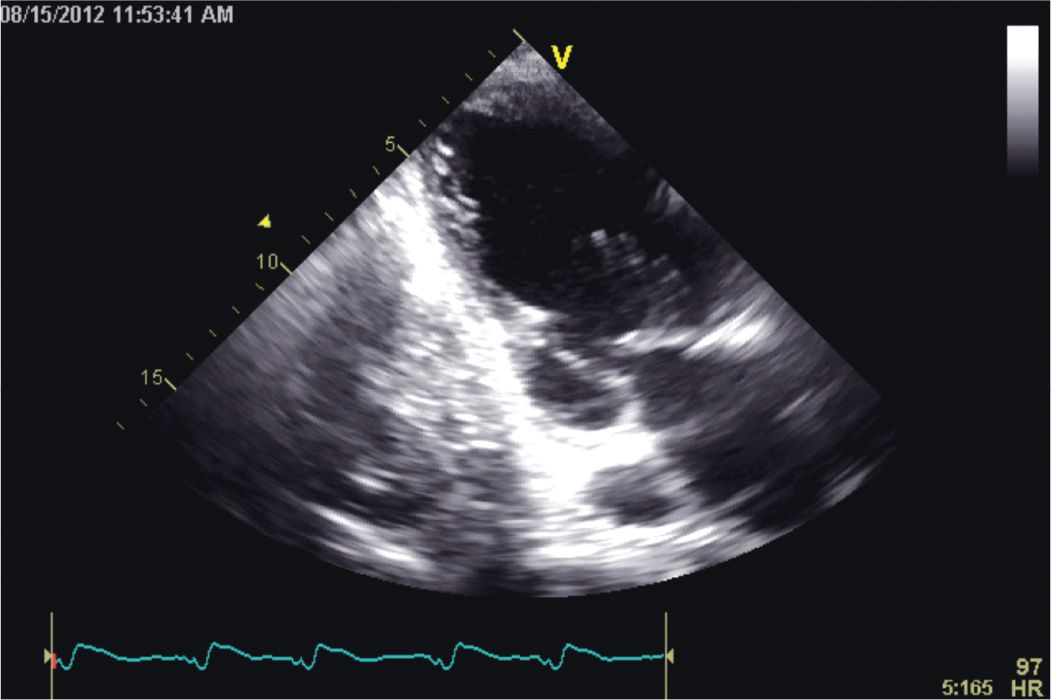
FIGURE 5-1-5 Apical 2 chamber image demonstrating akinesis and thinning of the inferior wall. A left pleural effusion is present.
OTHER DIAGNOSTIC TESTS AND PROCEDURES
• Electrocardiography: The ECG finding of pathological Q waves should strongly raise the suspicion of an ischemic etiology. The ECG, however, may be nonspecific in the absence of diagnostic Q waves; findings such as sinus tachycardia, nonspecific ST/T abnormalities, bundle-branch block, and conduction defects may be observed.
• Chest radiography is often nonspecific. The finding of cardiomegaly, with or without interstitial edema, is often observed. Pleural effusions may be observed in more advanced disease.
• Other imaging modalities that may be employed include nuclear medicine studies to evaluate for ischemia/viability, cardiac MRI, and cardiac PET. Cardiac catheterization is appropriate to define coronary artery anatomy and evaluate the patient for potential revascularization.
DIFFERENTIAL DIAGNOSIS
The clinical presentation of ischemic cardiomyopathy is relatively straightforward as the majority of patients have a prior history of CHD and frequently present with angina pectoris. The main differential is that of a dilated, nonischemic cardiomyopathy. Two points must be emphasized.
• The presence of asymptomatic, nonobstructive CHD does not prove causality unless prior infarction or significant hibernating/stunned myocardium is documented.
• Patients with nonischemic cardiomyopathies often present with chest pain symptoms that can resemble angina pectoris.
• Any number of other conditions can cause dilated cardiomyopathy. The history should focus on identification of specific etiologies (Table 5-1-1).
• The physical examination often reveals signs of heart failure. In some cases, discovery of an underlying systemic disease may be aided by the physical examination. In addition, the physical examination may help to exclude other potential causes of heart failure such as valvular heart disease or pericardial disease.
• In the setting of rapid supraventricular tachycardia or atrial fibrillation or flutter, a diagnosis of a tachycardia-mediated cardiomyopathy should be entertained.
TABLE 5-1-1 Causes of Dilated Cardiomyopathy
Toxins
Ethanol, cocaine
Chemotherapeutic agents
Medications
Trace elements
Metabolic
Nutritional
Infectious
Inflammatory
Neuromuscular disorders
Familial/genetic
Idiopathic
Ischemic
Stress-induced
Peripartum
Tachycardia-induced
LBBB dyssynchrony
DIAGNOSIS
• The diagnosis is made on the basis of clinical features, ECG findings, recognition of regional wall motion abnormalities and assessment of LVEF by echocardiography, and results of coronary angiography.
• Most patients present with a history of known CHD.
• While noninvasive testing may strongly suggest the diagnosis, coronary angiography is recommended for definitive diagnosis among patients with angina or significant ischemia potentially eligible for revascularization (Class I, LOE B).7 The extent of disease found at angiography is a better indicator of prognosis than is the clinical diagnosis of ischemic cardiomyopathy.1
• It must be reemphasized that in patients with reduced LVEF chest pain alone is not a sufficient diagnostic criterion since as many as one-third of patients with nonischemic cardiomyopathies may complain of this symptom. For this group of patients, coronary angiography is suggested due to common occurrence of false-positive findings on noninvasive testing.
MANAGEMENT
• Treatment with antiplatelet agents is recommended for all patients without contraindications.
• ACE-inhibitors and β-blockers are recommended for all patients with LV dysfunction (LVEF ≤40%). Management of patients with heart failure should follow that which is detailed in published guidelines.7
• While risk factor modification is recommended for all patients with ischemic heart disease and heart failure, the role of statins among patients with systolic heart failure (including those with ischemic cardiomyopathy) is less clear. The two largest randomized trials to date addressing this issue8,9 showed that initiating therapy with a HMG Co-A reductase-inhibitor was not useful among patients with systolic heart failure regardless of whether or not CHD was present.
• Per guideline recommendations, placement of ICDs is indicated for patients with appropriate indications.7
• Until recently, the role of coronary artery bypass grafting (CABG) surgery among patients receiving contemporary medical therapy was uncertain. In the recently published STITCH trial,10 patients with ischemic cardiomyopathy (LVEF <35%) and CHD amenable to bypass surgery were randomized to strategies that compared best medical therapy to surgical revascularization along with medical therapy. After a median follow-up of 56 months there was no difference in all cause mortality; patients undergoing CABG experienced lower rates of death from cardiovascular causes and hospitalizations for cardiac-related causes. A second publication from the STITCH group11 addressed the issue of whether or not surgical revascularization improved survival among patients with chronic ischemic LV dysfunction when viable myocardium is documented. This investigation has shown no survival benefit from revascularization in this population. It should be appreciated that patients with left main-stem disease or acute coronary syndromes were excluded from further analysis.
• Symptomatic patients with angina pectoris despite optimal medical therapy should be considered for revascularization. A trial of medical therapy may be appropriate in all other patients with revascularization held in reserve for those with progressive symptoms despite optimal medical therapy.
FOLLOW-UP
Patients should be followed regularly by a cardiologist to assess for adequacy of therapy and progression of underlying disease processes. Consultation with a heart failure specialist is prudent with significant disease progression despite adequate medical/device therapy and when more advanced therapies are being considered.
REFERENCES
1. Bart BA, Shaw LK, McCants CB Jr, et al. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol. 1997;30:1002-1008.
2. Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077-1084.
3. Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail. 2005;7:119-125.
4. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003;108:1146-1162.
5. Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society of Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2011;57(9):1126-1166.
6. Gorcsan J 3rd, Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. 2011;58:1401-1413.
7. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. This document is available on the World Wide Web sites of the American College of Cardiology (www.cardiosource.org) and the American Heart Association (my.americanheart.org).
8. Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261.
9. GISSI-HF investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231-1239.
10. Velazquez EJ, Lee KL, Deja MA, et al. STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607-1616.
11. Bonow RO, Maurer G, Lee KL, et al. STICH Trial Investigators. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617-1625.
CLINICAL CASE PRESENTATION
A 41-year-old man with Becker muscular dystrophy was referred for evaluation of cardiac performance due to progressively worsening dyspnea on exertion and bilateral lower extremity edema. Two years prior he was seen at an outside institution for evaluation of fatigue and cough. At that time, he was found to be in atrial fibrillation and to have a dilated LV, severe mitral regurgitation (MR), and an LVEF of 30%. Coronary angiography showed nonobstructive disease. He underwent electrical cardioversion, and an appropriate medical program was initiated. Subsequently, he developed episodes of nonsustained ventricular tachycardia, and an ICD was implanted. He denied paroxysmal nocturnal dyspnea and orthopnea and did not complain of chest pain. He did not complain of palpitations and had experienced no ICD discharges. Due to insurance issues, he could not afford several of his medications. He noted an approximate 10-pound weight gain since his last visit with a physician. An older brother died several years earlier from noncardiac complications of Becker muscular dystrophy. He did not smoke, consume alcohol, or use illicit drugs. He held part-time employment as a librarian. Echocardiography showed a dilated and hypocontractile LV (end-diastolic dimension 80 mm and end-systolic dimension 70 mm) with inferolateral akinesis and wall thinning; the LVEF was estimated to be 20%. The LA was dilated, and severe functional MR was present. The PA systolic pressure was estimated to be 60 mm Hg. The RV was dilated and hypokinetic.
CLINICAL FEATURES AND PRESENTATION
• Patients with nonischemic cardiomyopathies often present with manifestations of heart failure such as progressive exertional dyspnea, exercise intolerance, PND or orthopnea, or fluid retention/edema.
• Other symptoms such as chest pain, atrial or ventricular arrhythmias, conduction disturbances, thromboembolic events, or sudden cardiac death may also lead to clinical presentation.
• Patients with advanced disease can present with clinical features consistent with a low cardiac output syndrome that may include fatigue, anorexia, hepatic congestion, edema, cool extremities, and tachycardia.
• Patients may present with asymptomatic LV dysfunction discovered as cardiomegaly on chest imaging or a low LVEF on echocardiography obtained for another clinical indication.
• The physical examination may show signs of cardiac dysfunction such as narrowed pulse pressure, resting tachycardia, pulsus alternans, jugular venous distention, pulmonary rales, displaced apical impulse, gallop rhythm, and peripheral edema. A low cardiac output state is likely when a narrow pulse pressure (<25 mm Hg), cool extremities due to peripheral vasoconstriction, diaphoresis, and resting (sinus) tachycardia are found.
EPIDEMIOLOGY
• The estimated incidence of nonischemic dilated cardiomyopathy is 5 to 8 cases/100,000 persons per year. The prevalence is estimated to be 36 cases/100,000 population. Preclinical heart failure may be present in up to 50% patients with LV dysfunction.1
• The diagnosis is made most often among persons 20 to 60 years old, but individuals of any age can be affected. A slight male predominance is observed.
• Up to 20% to 35% of first-degree relatives may be affected (most subclinical), suggesting an underlying genetic susceptibility to disease.
PATHOPHYSIOLOGY AND ETIOLOGY
• Diverse causes of dilated cardiomyopathy are recognized (see Table 5-1-1).
• After excluding an ischemic etiology, almost 50% of cases are considered idiopathic.2 Other potential etiologies of nonischemic cardipomyopathy include a variety of infectious, toxic/metabolic, nutritional, genetic, and miscellaneous causes.
• Dilated cardiomyopathy is the most common form of cardiomyopathy. It is characterized by enlargement and impairment of systolic performance (ejection fraction ≤40%) of one or both ventricles.
![]() Often the LV assumes a more spherical geometry leading to papillary muscle displacement and functional MR.
Often the LV assumes a more spherical geometry leading to papillary muscle displacement and functional MR.
![]() Dilatation of the mitral annulus along with impaired LV systolic performance may also contribute to the occurrence of functional MR.
Dilatation of the mitral annulus along with impaired LV systolic performance may also contribute to the occurrence of functional MR.
![]() Concomitant diastolic dysfunction is common.
Concomitant diastolic dysfunction is common.
![]() Intracavitary thrombus (often layered and located at the LV apex) is seen frequently on postmortem examinations, but less often is documented by echocardiography.
Intracavitary thrombus (often layered and located at the LV apex) is seen frequently on postmortem examinations, but less often is documented by echocardiography.
• On histology, myocyte hypertrophy and interstitial fibrosis are characteristic findings. Infiltration with lymphocytes or giant cells may be observed with myocarditis.
• Survival is dependent on the etiology of the underlying disease process along with disease-independent factors such as LVEF, NYHA class, 6-minute walk distance, and maximal oxygen consumption on cardiopulmonary exercise testing.
ECHOCARDIOGRAPHY
The hallmark of a dilated cardiomyopathy is the presence of a dilated LV cavity with reduced systolic (and diastolic) performance (Figures 5-2-1 and 5-2-2).
• In comparison to an ischemic cardiomyopathy, LV systolic dysfunction often manifests in a pattern of global rather than regional wall motion abnormality.
• However, with nonischemic cardiomyopathies, regional variation in contraction can be observed commonly; often the basal inferolateral wall shows relatively preserved contractility in comparison to other wall segments. As illustrated by this case example, regional wall motion abnormalities may be seen in certain cases.
• An accurate assessment of LVEF is mandatory (biplane Simpson’s method or 3-D echocardiography are preferred) as patients with persisting LVEF <35% despite adequate medical therapy are candidates for prophylactic ICD placement.
![]() Wall thickness may be normal or reduced.
Wall thickness may be normal or reduced.
• With advanced disease, a more spherical LV geometry (ratio of long-axis/minor axis <1.5) may be seen (Figure 5-2-3).
• Dilatation of the LA, RV, and/or RV may be present.
• The LV apex should be scanned carefully for the presence of thrombus.
• The RV may exhibit reduced systolic performance due to imposed afterload (such as increased PA systolic pressure or pulmonary vascular resistance) and/or pathological involvement.
• The M-mode examination may show an increased E-point-septal separation distance (corresponding with reduced LV stroke volume), a B-bump (interrupted A-C closure) indicative of elevated LV end-diastolic (and LA) pressure, and gradual closure of the aortic valve echo during systole.
• Doppler assessment of diastolic function rarely is normal among patients with advanced nonischemic cardiomyopathies.
• Increased pulmonary artery systolic pressures and functional mitral regurgitation (Figures 5-2-4 and 5-2-5) are present in the majority of patients.
• Indicators of adverse prognosis on echocardiography include increased end-diastolic and end-systolic volumes, LVEF <40%, sphericity index <1.5, LV dp/dt <600 mm Hg/s, abnormal myocardial performance index (>0.4), a restrictive filling pattern, and increased left atrial volume index.
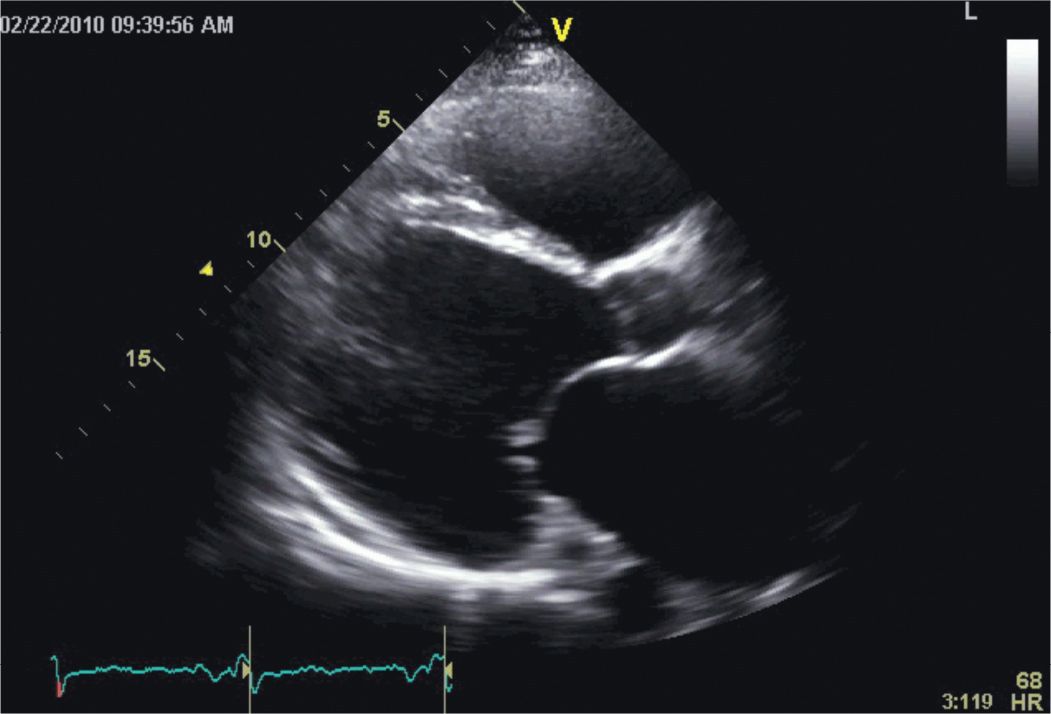
FIGURE 5-2-1 Parasternal long-axis view showing a massively dilated and diffusely hypocontractile LV in a patient with an advanced non-ischemic cardiomyopathy. As illustrated by this example, a regional wall motion abnormality is demonstrated; however, the majority of patients with nonischemic dilated cardiomyopathies do not manifest such a focal finding. Also note that both the left atrium and right ventricle are dilated, mitral leaflet excursion is reduced, and the coronary sinus is prominent.
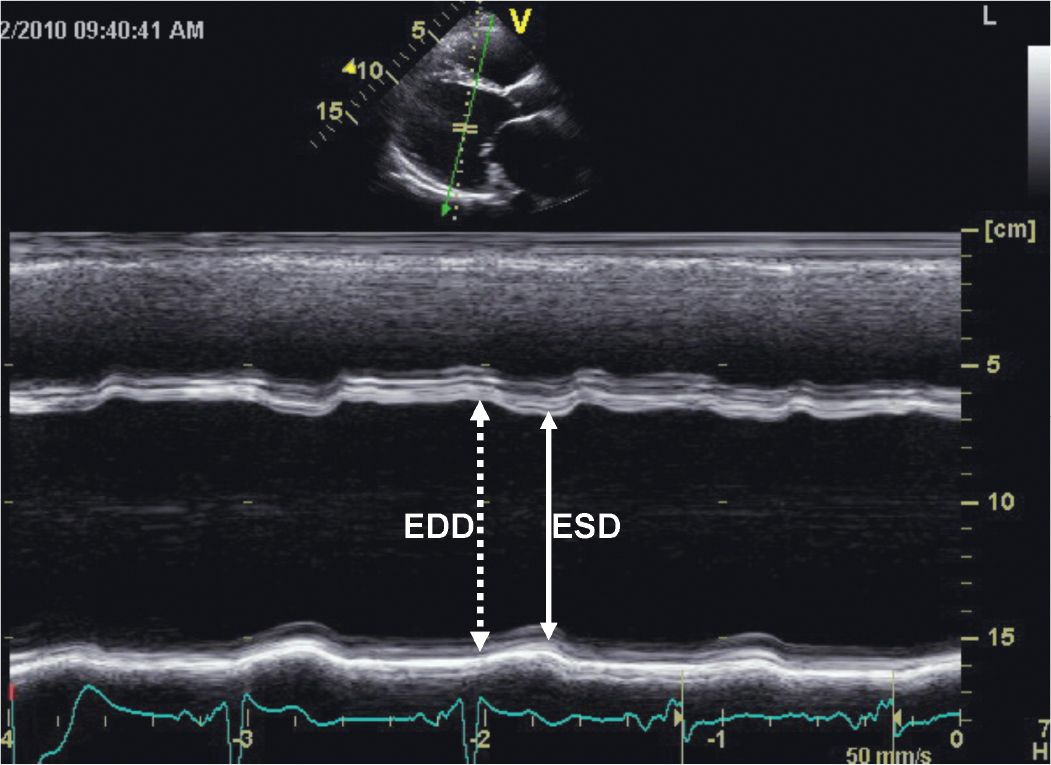
FIGURE 5-2-2 M-mode recording demonstrating the findings of a dilated and hypocontractile LV. EDD = end-diastolic dimension; ESD = end-systolic dimension.
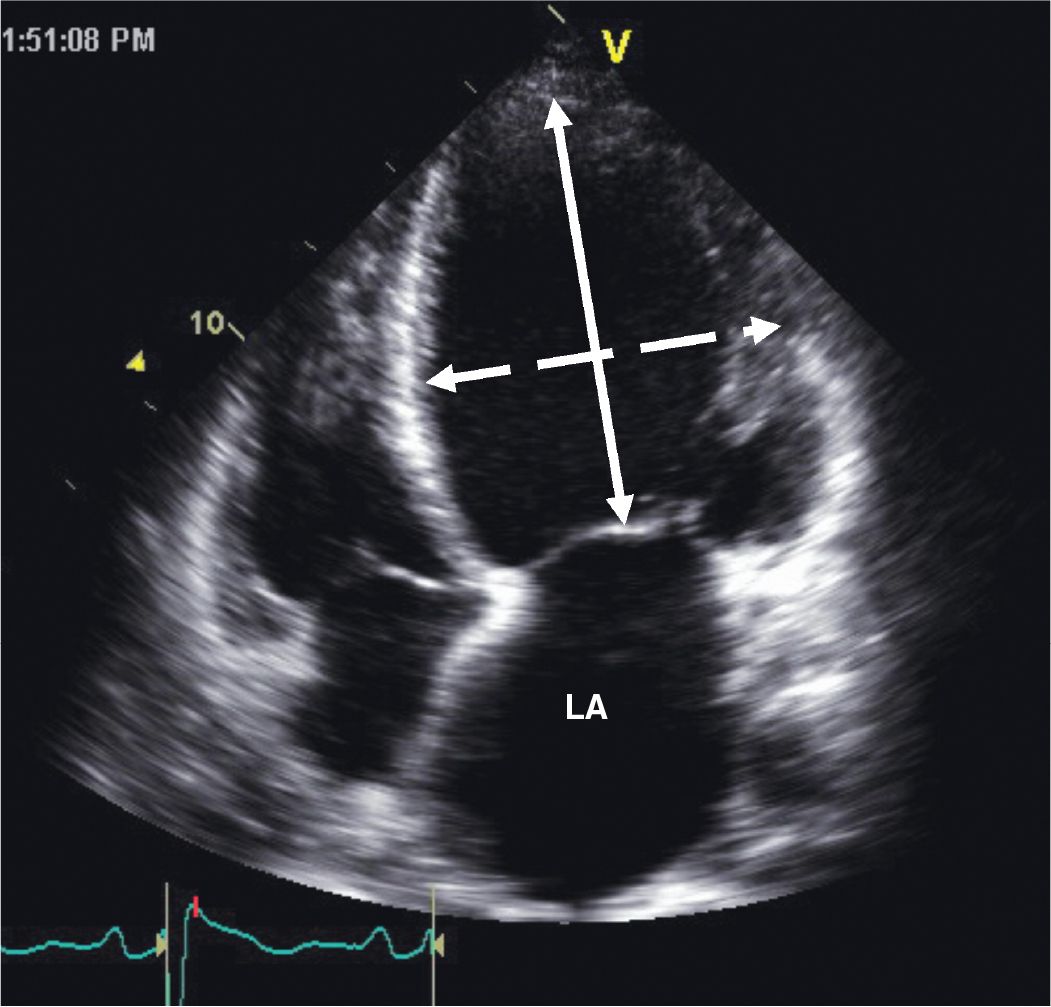
FIGURE 5-2-3 Apical 4 chamber view illustrating adverse geometric remodeling in a patient with nonischemic cardiomyopathy. The LV has assumed a more spherical geometry as illustrated by a reduced sphericity index, ratio of major-axis dimension (solid arrow) to minor-axis dimension (stippled arrow), of 1.33 in this example. Note also the dilatation of the left atrium (LA).
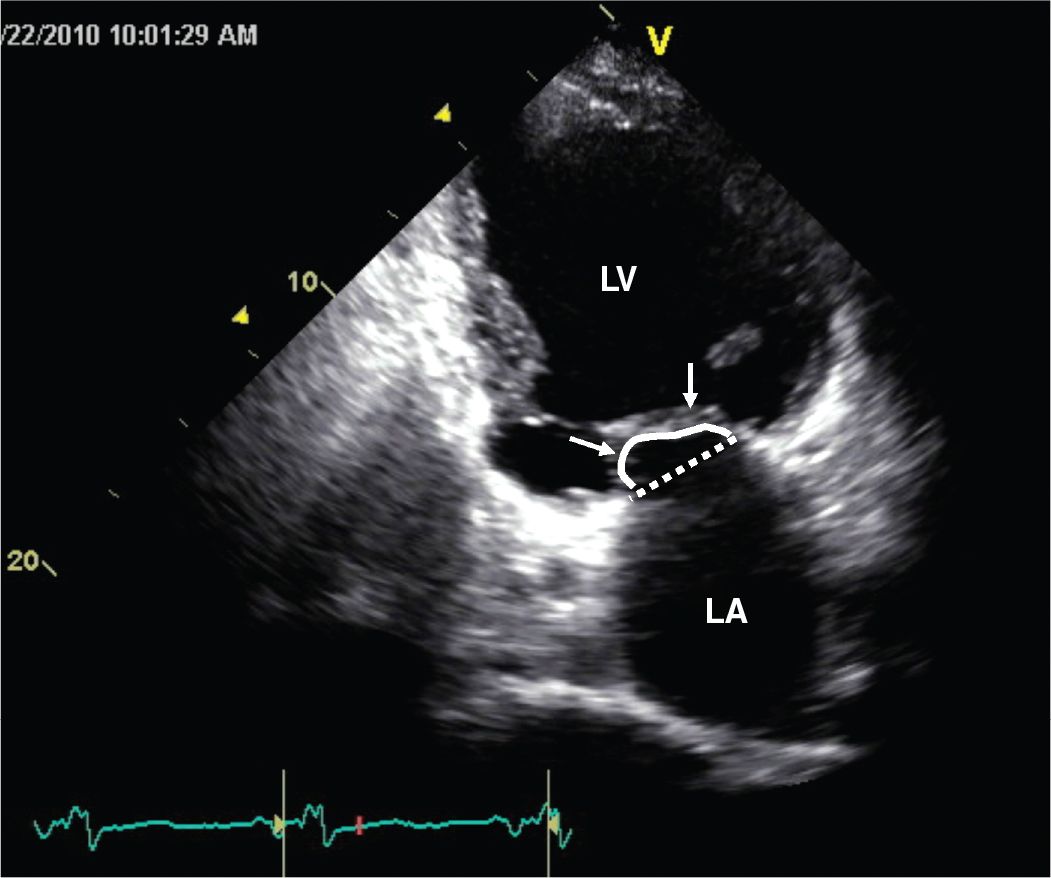
FIGURE 5-2-4 Apical 2 chamber view recorded in systole illustrating adverse LV geometrical remodeling that results in papillary muscle displacement and failure of the mitral leaflets to coapt at the plane of the mitral annulus (dotted line). This apical displacement (“tenting”) of the leaflets (arrows) can be quantified by assessing the area encompassed between the solid line and the annular plane.
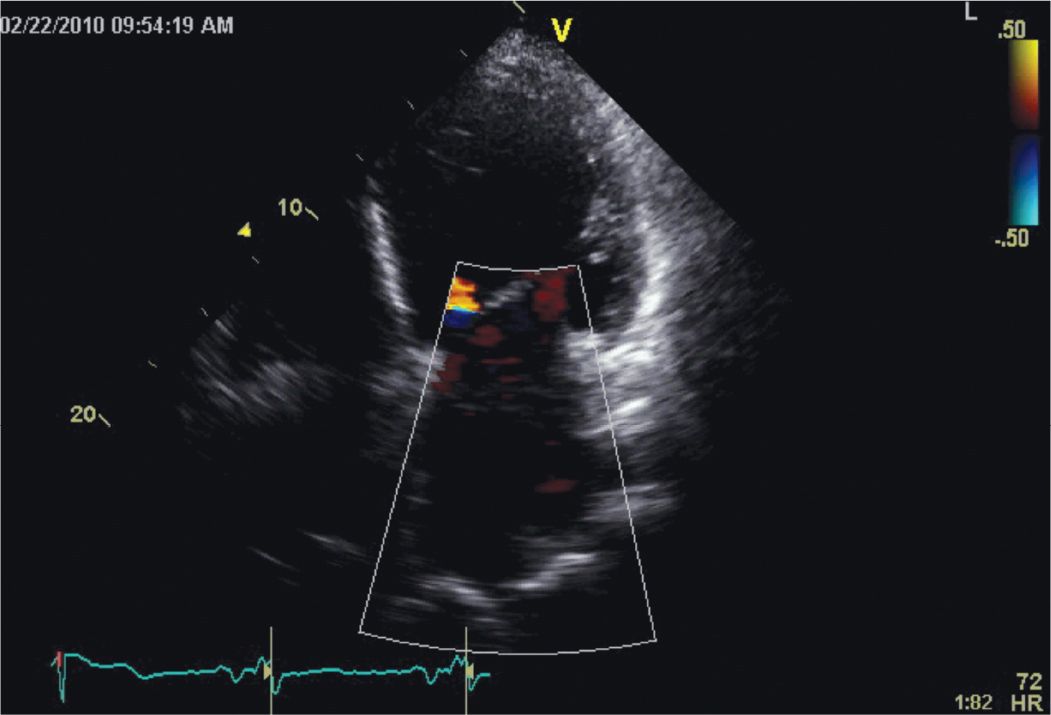
FIGURE 5-2-5 This apical 4 chamber view illustrates the presence of severe functional mitral regurgitation due to papillary muscle displacement occurring as consequence of the adverse LV remodeling.
OTHER DIAGNOSTIC TESTS AND PROCEDURES
• As a number of conditions may potentially result in a nonischemic cardiomyopathy, the history should focus on identification of specific etiologies (see Table 5-1-1).
• The physical examination often reveals signs of heart failure. In some cases, discovery of an underlying systemic disease may be aided by the physical examination. In addition, the physical examination may help to exclude other potential causes of heart failure such as valvular heart disease or pericardial disease.
• Appropriate biochemical tests and serologies should be obtained as indicated.
• The ECG is often non-specific; findings such as sinus tachycardia, non-specific ST/T abnormalities, bundle-branch blocks, and conduction defects may variably be observed. A finding of pathological Q waves should raise the suspicion of possible ischemic etiology.
• In the setting of supraventricular tachycardia or atrial fibrillation or flutter, a diagnosis of a tachycardia-mediated cardiomyopathy should be entertained.
• Chest radiography findings are often nonspecific. Cardiomegaly, with or without interstitial edema, may be observed. Pleural effusions are often observed in more advanced disease.
• Other imaging procedures such as myocardial perfusion imaging to assess for possible ischemia and viability and CMR to evaluate for entities such as arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D), myocarditis, or sarcoidosis should be employed as indicated.
• Coronary arteriography should be used to exclude CHD when complaints of chest pain exist, with the finding of regional wall motion abnormalities suggestive of ischemic heart disease on echocardiography, or with findings suggestive of an ischemic etiology on stress perfusion imaging.
• Endomyocardial biopsy is not routinely recommended. In general, this procedure is reserved for patients with dilated cardiomyopathy and new onset heart failure associated with hemodynamic compromise or electrical instability. Specific instances where cardiac biopsy may prove useful include distinguishing lymphocytic from giant cell myocarditis, grading the severity of anthracycline-induced cardiomyopathy, and in diagnosing disorders such as amyloidosis and sarcoidosis.
DIAGNOSIS
The diagnosis is usually made on the basis of clinical history, findings on physical examination, and comprehensive echocardiography. In some cases, additional testing such as coronary angiography or CMR may be required. Endomyocardial biopsy may be required for definitive diagnosis in highly selected cases.
MANAGEMENT
• For patients with symptomatic heart failure (ACC/AHA stages C and D), provision of standard heart failure therapy as detailed in the ACC/AHA HF guideline3 is indicated.
• For patients with CHD, valvular heart disease, or uncorrected congenital defects, percutaneous intervention or surgery as appropriate is indicated.
• Patients with alcohol-induced cardiomyopathy should abstain from alcohol completely.
• Medical therapy and/or ablation of atrial arrhythmias are indicated for patients with tachycardia-induced cardiomyopathy.
• Device therapy (CRT, ICD) should be used when appropriate clinical indications exist.
• For selected patients with advanced disease, mechanical support, as a temporizing measure or destination therapy, or cardiac transplantation may be indicated.
• For patients with asymptomatic LV dysfunction (LVEF ≤40%), ACC/AHA Stage B heart failure, attention should be paid to reducing the risk of disease progression by modifying risk factors for development of cardiovascular disease. Administration of ACE-inhibitors and/or β-blocker is indicated in the absence contraindications to their use.
FOLLOW-UP
Close follow-up with a clinical cardiologist or heart failure specialist is suggested depending upon the stage of the disease. Clinical screening of first-degree family members of patients with idiopathic dilated cardiomyopathy is suggested as 20% to 35% of these family members may be affected.4
REFERENCES
1. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194-202.
2. Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077-1084.
3. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. This document is available on the World Wide Web sites of the American College of Cardiology (www.cardiosource.org) and the American Heart Association (my.americanheart.org).
4. Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:1641-1649.
SECTION 3
CLINICAL CASE PRESENTATION
A previously healthy 37-year-old woman presented to hospital with complaints of increasing fatigue and having had progressive orthopnea and paroxysmal nocturnal dyspnea over the prior 2 weeks. About 12 weeks earlier, she delivered a healthy child after an uncomplicated full-term delivery. In the emergency department she was found to have a blood pressure of 70/40 mm Hg, pulse rate of 120 beats/min, and respiratory rate of 20 breaths/min; she was afebrile. Jugular venous distention was present along with decreased bibasilar breath sounds and crepitant pulmonary rales. Her heart rhythm was regular, and biventricular gallop sounds were heard. A grade II/VI holosystolic murmur was heard at the apex, and a grade II/VI holosystolic murmur was heard at the left lower sternal border. Bilateral ankle edema was present. A 12-lead ECG showed sinus tachycardia with frequent ventricular premature contractions. Chest radiography demonstrated cardiomegaly, bilateral pleural effusions, and intersti tial edema. A lactate level was measured at 5.1 mmol/L (upper limit of normal = 2.4 mmol/L). Her BNP level was 2540 pg/mL. Cardiac troponin I levels were in the normal range. Thyroid function tests were normal. Two-dimensional echocardiography (Figure 5-3-1) showed severe biventricular dysfunction with an estimated LVEF of 18%, biatrial enlargement, moderate mitral regurgitation, moderate to severe tricuspid regurgitation, a PASP estimated to be 30 mm Hg plus right atrial pressure, and a dilated inferior cava. Supportive care, including furosemide, dopamine, and milrinone drips, was started. Over the ensuing 48 hours she experienced improved hemodynamic status and excellent urine output. The intravenous medications were able to be weaned off, and she was started on an appropriate oral heart failure regimen. On follow-up echocardiography performed 3 months following hospital dismissal, her LVEF was 40% to 45%; echocardiography performed at 18 months (Figure 5-3-2) showed her LVEF to be 55% and the valvular insufficiency had resolved. She remains on an ACE-inhibitor and β-blocker.
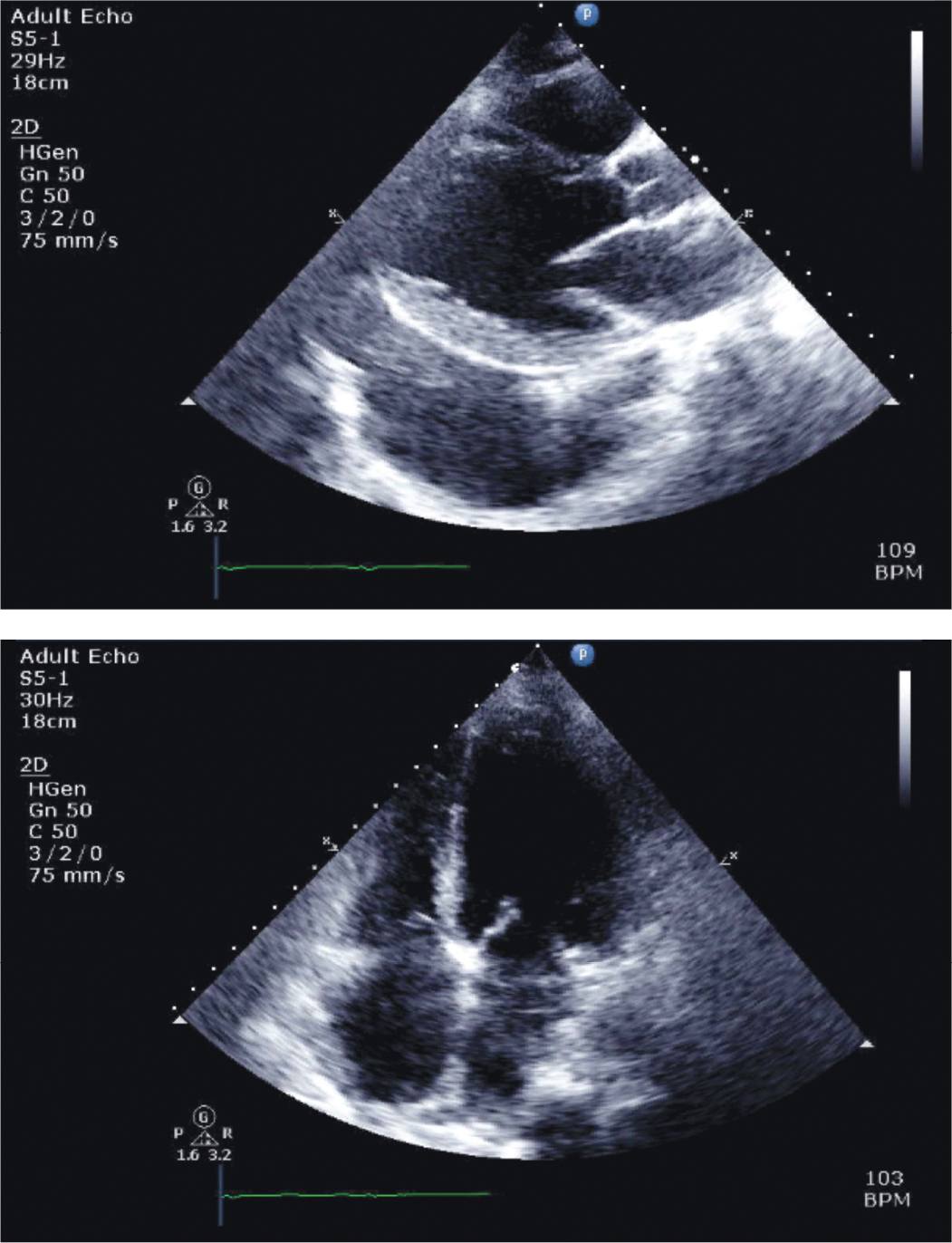
FIGURE 5-3-1 Parasternal long-axis (top panel) and apical 4 chamber (bottom panel) views of echocardiography performed on the day of presentation in the patient described in the case vignette. Note the presence of diffuse, severe hypokinesis of the LV, reduced RV function, and biatrial enlargement. A large left pleural effusion is seen on parasternal long-axis imaging.
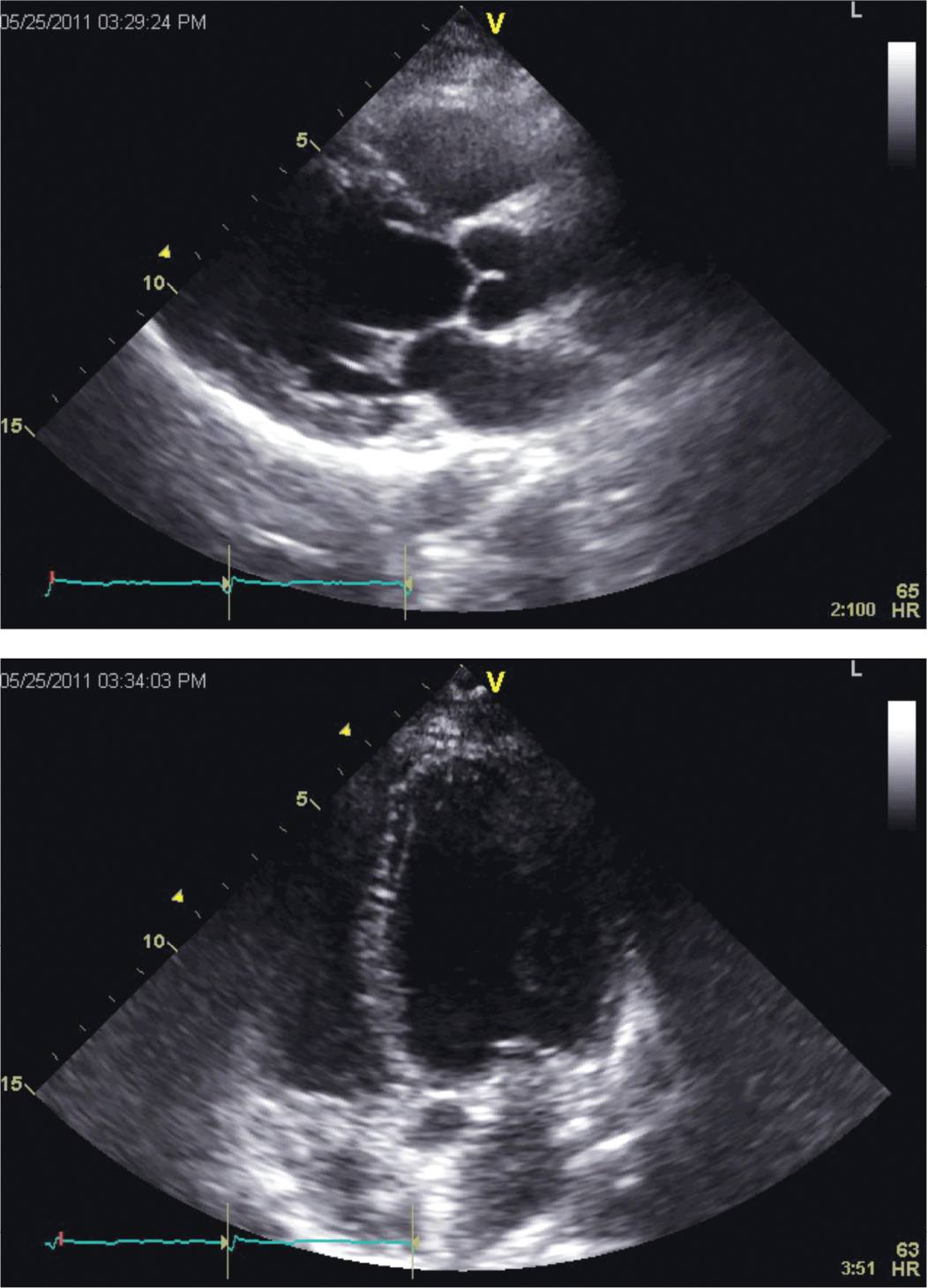
FIGURE 5-3-2 Parasternal long-axis (top panel) and apical 4 chamber (bottom panel) views from follow-up echocardiography obtained about 21 months after initial presentation. Normalization of LV and RV systolic function and atrial sizes is demonstrated.
CLINICAL FEATURES
Peripartum cardiomyopathy (PPCM) is a cause of pregnancy-associated heart failure occurring among women without previously known cardiovascular disease.
• Most patients present with heart failure symptoms and reduced LVEF (<45%) that develop in the last month of pregnancy, or more commonly, within the first 5 to 6 months following delivery (about 78% present with symptoms within 4 months of delivery).
• A more encompassing definition of this syndrome has been proposed recently by the Heart Failure Association of the European Society of Cardiology Working Group on PPCM.1
• This condition often presents insidiously; a high-index of suspicion is required as ankle edema, shortness of breath, and fatigue are frequent complaints occurring in the peripartum period.
• Other presenting complaints may include cough, PND, orthopnea, hemoptysis, abdominal discomfort due to liver congestion, palpitations, and dizziness. Patients most often present with NYHA Class III or IV symptoms.1
• Systemic or pulmonary embolism may bring a patient to clinical attention as thromboembolism is more frequent with PPCM than with dilated cardiomyopathies of other etiologies.
• The physical examination is similar to that of other dilated cardiomyopathies.
• The clinical course can be highly variable; spontaneous and complete recovery of LV function may occur (45%-78% by 6 months); however, some may be left with persisting LV dysfunction. Rapid progression with marked hemodynamic instability, ventricular arrhythmia, and development of end-stage heart failure may be an outcome in a subset of patients.
EPIDEMIOLOGY
As emphasized by the European Working Group, little is known about the true incidence of PPCM.1
• The incidence appears to vary by geographical locale ranging from 1:300 live births in Haiti to 1:1500-4350 live births in the United States.1,2
• Over time, the incidence of PPCM in the United States appears to be rising and may be tied to factors such as increasing maternal age, increased incidence of multi-fetal pregnancy, and increased disease recognition.2
• In the United States, the incidence of PPCM is reported to be highest among African American women and lowest in Latinas.
• The annual incidence of PPCM in the United States is estimated to be about 1350 cases.2
PATHOPHYSIOLOGY AND ETIOLOGY
The underlying mechanism of disease remains unknown. Several putative processes have been postulated as contributing factors in the pathogenesis.
• Pregnancy-related factors (age, multiparity, multifetal pregnancy).
• Presence of established risk factors for cardiovascular disease.
• Viral myocarditis, inflammation, and autoimmune responses.
• Genetic susceptibility and environmental risk factors are postulated to also play a role.
• Recent research has identified enhanced oxidative stress leading to activation of cathepsin D in cardiomyocytes and subsequent cleavage of the hormone prolactin into an angiostatic and pro-apoptotic 16 kDa fragment.3
• Suppression of prolactin production by bromocriptine has been shown to prevent the onset of PPCM in a mouse model.3
ECHOCARDIOGRAPHY
Echocardiography is an essential tool in helping establish the diagnosis of PPCM and excluding other possible diagnoses of myocardial dysfunction. By definition, the LVEF should be <45% and/or the fractional shortening (FS) should <30%.4 In addition, the LV end-systolic dimension should be >2.7 cm/m2.
• In a recent study, the mean LVEF on presentation was 29±11%.5
• Fractional shortening <20% and LV end-diastolic diameter >60 mm has been associated with a greater than 3-fold risk for persistent LV dysfunction.6
• Diastolic function abnormalities are the norm, and a restrictive filling pattern is common.
• Abnormal RV systolic performance and dilatation may be present and are encountered with greater frequency in PPCM compared to other causes of dilated cardiomyopathy.7
• Other findings may include dilatation of the atria, functional MR and TR, and pericardial effusions.
• Ventricular thrombus is reported to be present in 10% to 17% of cases.2
OTHER DIAGNOSTIC TESTING AND PROCEDURES
• Electrocardiography often exhibits nonspecific findings, but is rarely normal in PPCM. Sinus tachycardia, nonspecific ST/T abnormalities, voltage criteria for LVH, left bundle-branch block, and LA enlargement can be observed.
• The chest x-ray typically shows cardiomegaly and pulmonary congestion/edema. Pleural effusions may be present.
• Levels of NT-proBNP are reported to be markedly elevated in patients with PPCM on presentation and significantly higher in the patients who did not improve LV function at 6 months.8
• Cardiac magnetic resonance imaging has had limited study in patients with PPCM. In addition to a dilated and hypocontractile LV, other findings may include the presence of mural thrombus and evidence of fibrosis. While some reports have documented the occurrence of late gadolinium enhancement in PPCM, others have not.9 Guidelines recommend that gadolinium be avoided during pregnancy and that breast feeding not be performed for 24 hours after IV administration.10
• Other diagnostic tests and procedures such as right and left heart catheterization, endomyocardial biopsy, and assessment of viral titers are generally not indicated.
DIFFERENTIAL DIAGNOSIS
The diagnosis of PPCM is one of exclusion. The diagnosis is suggested by a carefully performed history and physical examination and often confirmed on the basis of increased BNP levels and echocardiography while excluding other potential causes of LV dysfunction in this patient population.
• Important alternative diagnoses to consider include aggravation or unmasking of a preexisting dilated cardiomyopathy, structural heart diseases such as preexisting congenital heart disease, valvular heart disease, or hypertensive heart disease, HIV/AIDS cardiomyopathy, pulmonary vascular disease, coronary heart disease, and pulmonary embolism.
MANAGEMENT
As the majority of patients present with NYHA Class III/IV symptoms, initial therapy is often directed at treating acute decompensated heart failure.
• Standard heart failure therapies, including administration of diuretics, renin-angiotensin-aldosterone modifying agents, and β-blockers, are indicated.
• For patients yet to deliver use of ACE-inhibitors and ARBs is contraindicated; hydralazine and nitrates can be used safely during pregnancy.
• Among patients with severe LV dysfunction (LVEF <30%), the use of anticoagulants should be considered due to the substantial risk of the presence of LV/RV thrombus.
• Coordination of care with a specialist in high-risk obstetrics is advised for pregnant patients (about 9% of patients with PPCM).1
• For patients with severe hemodynamic compromise, positive inotropic and/or pressor agents should used as dictated by the prevailing hemodynamics. Support with an intraaortic balloon counterpulsation device (IABP) may be required in selected cases. Inotropic agent or IABP-dependent patients may require further support with a ventricular assist device. Cardiac transplantation may be required if these therapies prove ineffective.
• An emerging, still experimental treatment is the addition of bromocriptine to standard heart failure therapy.1,11
PROGNOSIS
It should be noted that prognosis appears to vary by geographical location.1
• In the United States, recovery of LV function (LVEF ≥50%) is reported to occur in 45% to 78% of patients within 6 months.2
• Recovery of LV function may be lower among African American women compared with Caucasians.2
• Predictors of recovery include an LVEDD <55 mm, LVEF >30% to 35%, absence of LV thrombus, lower NT-pro BNP levels, and non-African American ethnicity.
• Mortality among US patients with PPCM is reported to range from 0% to 19%.2
FOLLOW-UP
As stated above, many US patients experience recovery of LV function within 6 months of diagnosis.
• Patients with residual LV dysfunction require ongoing follow-up and treatment with standard heart failure therapies per the ACC/AHA heart failure guideline recommendations.12
• Controversy exists over whether or not to withdraw heart failure medications among patients who experience recovery of LV function.2
Limited data exist to give formal recommendations regarding future pregnancy.
• It is clearly advised that patients with persisting LV dysfunction be strongly advised against future pregnancy.1,2,13
• Given limited data, the risk of future pregnancy among patients who recover LV function cannot be predicted accurately. It is recommended that these patients should be advised that future pregnancy may cause detrimental effects on cardiac function with attendant risks of worsening heart failure and possibly maternal and/or fetal death.13
REFERENCES
1. Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767-778.
2. Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659-670.
3. Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589-600.
4. Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94:311-316.
5. Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111:2050-2055.
6. Chapa JB, Heiberger HB, Weinert L, Decara J, Lang RM, Hibbard JU. Prognostic value of echocardiography in peripartum cardiomyopathy. Obstet Gynecol. 2005;105:1303-1308.
7. Karaye KM. Right ventricular systolic function in peripartum and dilated cardiomyopathies. Eur J Echocardiogr. 2011;12:372-374.
8. Forster O, Hilfiker-Kleiner D, Ansari AA, et al. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2008;10:861-868.
9. Mouquet F, Lions C, de Groote P, et al. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur Radiol. 2008;18:2765-2769.
10. Webb JA, Thomsen HS, Morcos SK. Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur J Radiol. 2005;15:1234-1240.
11. Sliwa K, Blauwet L, Tibazarwa K, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121:1465-1473. Erratum in: Circulation. 2010;121:e425.
12. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-239. This document is available on the World Wide Web sites of the American College of Cardiology (www.cardiosource.org) and the American Heart Association (my.americanheart.org).
13. Elkayam U, Tummala PP, Rao K, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567-1571 Erratum in: N Engl J Med. 2001;345:552.
CLINICAL CASE PRESENTATION
A 74-year-old woman with hypothyroidism presented to an outside hospital after experiencing a single episode of syncope. She was active and otherwise healthy; she had never before experienced syncope. On the day of presentation she felt a sense of generalized weakness and lightheadedness and then passed out while taking a shower. She recovered consciousness quickly, and a witness did not observe any seizure-like activity. She suffered bruising to her right knee. The patient denied chest discomfort, shortness of breath, nausea or vomiting, diaphoresis, or palpitation. Upon further questioning, she expressed concern about a recent economic downturn and its impact on her family-run business.
At the outside hospital, a 12-lead ECG showed ST-segment elevations in leads I, avL, and V1-V3. She was transferred for consideration of emergent coronary intervention. Her cardiac troponin I (cTnI) was 3.8 ng/mL (normal <0.03) and CK-MB was 18.7 ng/dL (normal <4.4). Prior to angiography, an urgent echocardiogram showed evidence of severe LV dysfunction; the basal segments of all walls showed preserved to increased contractility while the midapical segments of all walls were akinetic. The LVEF was estimated visually as being 30%.
Coronary angiography showed only minor luminal irregularities throughout the coronary tree. Appropriate heart failure medications were initiated. Over the ensuing 24 hours, the cTnI peaked at 9.3 ng/mL. Follow-up ECG revealed resolution of the ST-segment elevations with development of new T wave inversions in the anterolateral leads along with QT interval prolongation. The patient remained asymptomatic, and no rhythm disturbances were documented while she was monitored.
Echocardiography prior to hospital dismissal showed only mild mid-to-apical anteroseptal hypokinesis. Within 2 weeks of discharge her LV function normalized fully, and by 2 months the ECG abnormalities resolved. She has had no recurrent symptoms over the past several years.
CLINICAL FEATURES
Stress-induced (tako-tsubo) cardiomyopathy is a clinical syndrome characterized by transient and often severe LV systolic dysfunction that frequently is presaged by concomitant physical or emotional stressors.
• An identifiable “trigger” may not be apparent in 11% to 30% of cases.
• While this syndrome most commonly occurs in postmenopausal women, recent reports reveal men and younger (premenopausal) women may account for 10% to 30% of cases.
• The presentation can be quite similar to that of an acute coronary syndrome with chest pain and ECG abnormalities that mimic acute myocardial infarction.1 Other presenting complaints may include dyspnea or syncope.
• This syndrome occurs most commonly among post-menopausal women and is reported to account for up to 2% of urgent coronary angiography performed for presumed acute coronary syndromes.2
• Coronary angiography typically shows minimal disease or disease that cannot explain the diffuse nature of the wall motion abnormalities seen on echocardiography or contrast ventriculography.
• As described originally,3 wall motion abnormalities involving the mid-distal LV while sparing the basal segments led to development of a peculiar ventricular morphology (Figure 5-4-1) resembling that of a “tako-tsubo” (Japanese octopus trap).
• Recent reports, however, show that variants of this syndrome with wall motion abnormalities involving only the midventricluar wall segments or the basal-midventricle with sparing of the apex (“inverted tako-tsubo”) occur in 18% to 25% of cases.4
• Involvement of the RV is seen in about one-third of cases5 (Figure 5-4-2).
• In addition to patients presenting with symptoms mimicking acute coronary events, this syndrome in our experience often occurs in critically ill patients in intensive care units and may manifest as heart failure, ECG abnormalities, and mild elevations of cardiac biomarkers.
• The majority of patients experience a relatively benign in-hospital course, and rapid recovery of LV function is expected. Delay in recovery of LVEF for up to 12 months is reported to occur in about 5% of patients.6
• Ventricular cavitary thrombus may be seen in 5% of patients.6
• Some patients may experience severe heart failure symptoms requiring intravenous diuretics.
• A subset of patients presents with severe hemodynamic compromise due to pump dysfunction, ventricular arrhythmias, or LV outflow tract obstruction can be seen. Such patients may require supportive therapy with mechanical ventilation, inotropic or vasopressor agents, IABP, or antiarrhythmic agents depending upon the clinical circumstances.
• The in-hosptial mortality rate is reported to be about 2%.
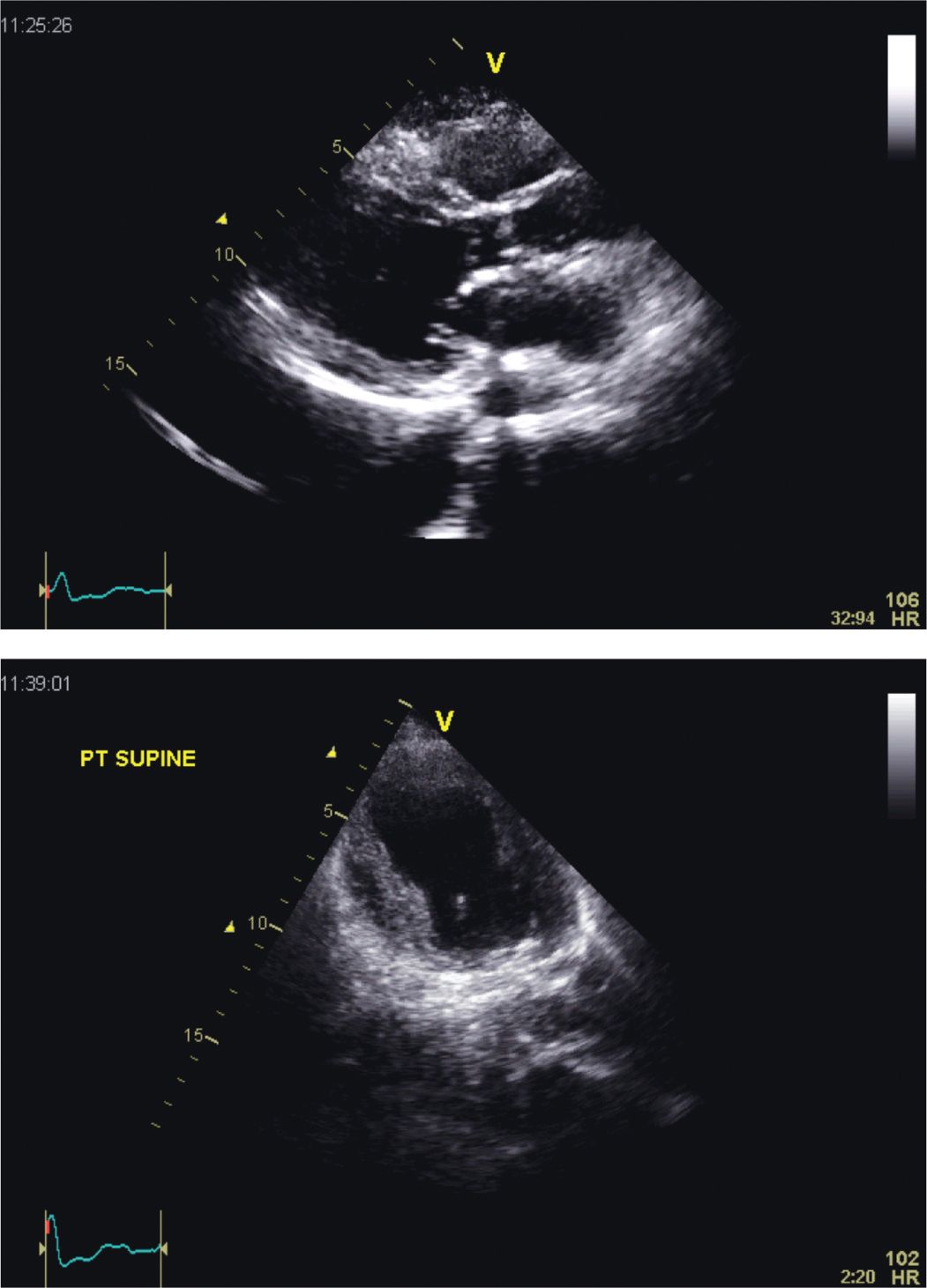
FIGURE 5-4-1 Parasternal long-axis (top panel) and apical 4 chamber (bottom panel) views in a patient with the classic form of stress-induced (“Tako-tsubo”) cardiomyopathy. In this example, only the basal segments of the left ventricular (LV) walls contract; the remaining walls segments are shown to be akinetic. The extensive nature of the LV wall motion abnormality observed is not consistent with involvement of a single coronary artery flow distribution.
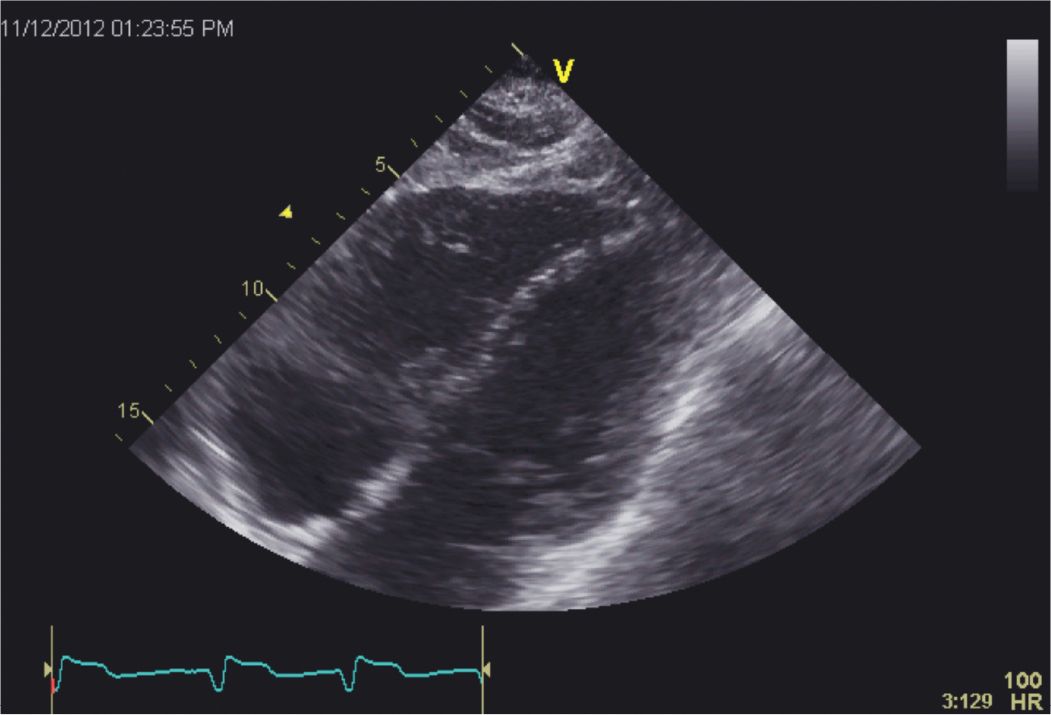
FIGURE 5-4-2 Subcostal 4 chamber view demonstrating akinesis of the mid and distal portions of the diaphragmatic wall of the right ventricle (RV) in a patient with stress-induced cardiomyopathy. Approximately 25% to 30% of patients with stress cardiomyopathy are shown to have RV involvement.
ECHOCARDIOGRAPHY
The classic finding is that of wall motion abnormalities not confined to a single epicardial coronary artery territory, typically involving the mid-distal LV. Variants of the “classic” pattern of apical ballooning can be recognized (Figure 5-4-3) readily. The typical findings on echocardiography can be seen with other imaging modalities such as contrast ventriculography or CMR.
• Echocardiography may also reveal other findings including valvular dysfunction such as mitral regurgitation, intracavitary thrombus located in proximity to akinetic wall segments, and evidence of RV involvement.
• An important application of echocardiography in this syndrome is assessment of the patient with hemodynamic compromise. While poor pump function accounts for the majority of such patients, recognition of LV outflow tract obstruction due to systolic anterior motion of the anterior mitral leaflet (10%-15% of such patients) is important and may prompt an alteration in the therapeutic plan.
• Because of its utility in performing serial evaluation, echocardiography is ideally suited for follow-up of these patients in order to document resolution of LV dysfunction.
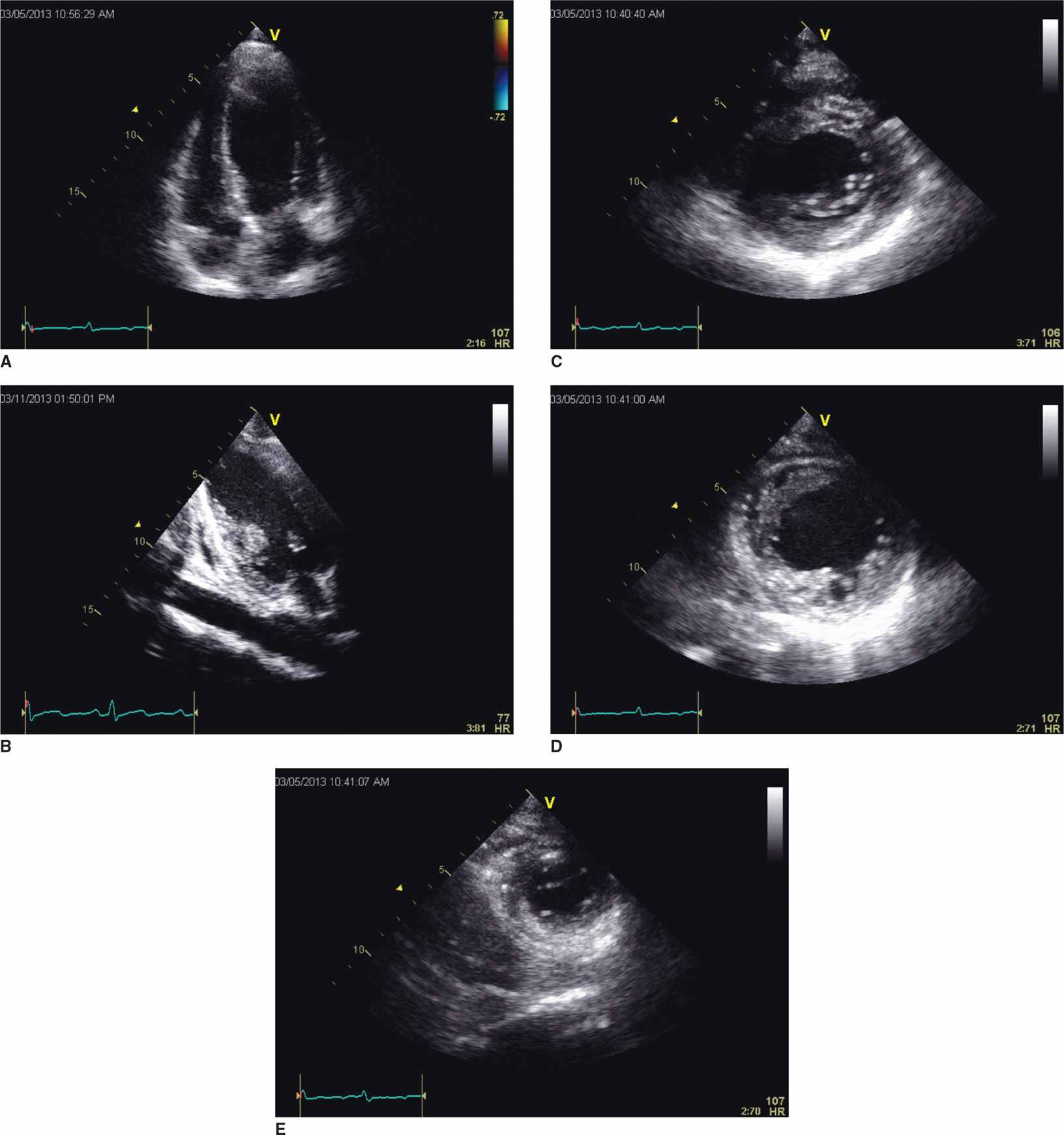
FIGURE 5-4-3 Mid-ventricular variant form of stress-induced cardiomyopathy. In this example, imaging in the apical 4 (A) and 2 chamber (B) planes demonstrates preserved contractility of the apical and basal portions of the LV with akinesis of the mid segments of the ventricular walls. Short-axis slices at the basal (C), mid (D), and apical (E) portions of the left ventricle further demonstrate the distinct regionality of wall motion abnormality in this variant form of stress-induced cardiomyopathy.
OTHER DIAGNOSTIC TESTING
Coronary Angiography
• Patients often undergo urgent catheterization as their clinical presentation, and ECG findings often mimic an acute coronary syndrome. The diagnosis is often apparent after excluding significant epicardial CAD on coronary angiography.
Electrocardiography
• May show a spectrum of findings ranging from initially normal ECG to ST-segment elevation (most common presentation), nonspecific ST/T abnormalities, Q waves, and diffuse T-wave inversion with prolonged QT interval.
• During recovery, greater than 80% of patients manifest diffuse T-wave inversion.7
Cardiac troponin levels are increased, but less so than with acute myocardial infarction.
CMR has been used to aid in making this diagnosis:
• Similar to other imaging modalities, wall motion abnormalities in a distribution not explained by that of single epicardial coronary artery involvement are observed.
• In a recent study, Eitel et al.,5 reported that 82% of patients with stress cardiomyopathy exhibited the “classic” pattern of apical ballooning, 17% had involvement limited to midventricular wall segments, and 1% had basal-only involvement (“reverse tako-tsubo”).
• In addition to defining wall motion abnormalities, CMR late gadolinium enhancement is usually absent with stress cardiomyopathy in comparison to acute myocardial infarction.
• Myocardial edema may be found in patients with stress cardiomyopathy, but this is a nonspecific finding also seen with myocarditis or acute myocardial infarction.
PATHOPHYSIOLOGY/ETIOLOGY
The precise etiologic mechanism remains unknown.
• Most episodes are preceded by an identifiable physical or emotional stressor.
• At present, catecholaminergic excess, either by causing direct myocardial toxicity or microvascular spasm, appears to be the most plausible mechanism. Catecholamoine levels in patients with stress cardiomyopathy have been documented to be higher than in patients with acute myocardial infarction in Killip class III.8
• Endomyocyocardial biopsy results obtained in a small number of patients with stress cardiomyopathy revealed changes consistent with a direct catecholamine effect on the myocardium.
• It must be emphasized that stress cardiomyopathy appears to be a heterogeneous clinical syndrome and likely no single mechanism can be advanced as an explanation for all cases.
The predilection for mid-apical LV cavity involvement is also not explained.
• A number of mechanisms such as multivessel coronary artery spasm, LAD plaque rupture, and microvascular dysfunction have been postulated as causes of this syndrome, but these purported mechanisms find little support.
DIAGNOSIS
The diagnosis is usually made by recognition of the clinical setting (often occurring among postmenopausal women with antecedent physical or emotional stress) and by exclusion of an acute coronary syndrome given that presentation with chest pain and ECG changes is common. Most patients undergo coronary angiography and contrast ventriculography. The recognition of substantial wall motion abnormalities not in the typical distribution of a coronary supply territory and with nonobstructive disease suggests the diagnosis. Typically, the rise in biomarkers of myocardial injury (cardiac troponin) is relatively minor given the extent of ECG changes and wall motion abnormality observed. Diagnostic criteria have been proposed by investigators at the Mayo Clinic.1
• Transient LV wall motion abnormalities involving the apical and/or midventricular myocardial segments with wall motion abnormality extending beyond a single epicardial coronary distribution.
• Absence of obstructive epicardial CAD or angiographic evidence of plaque rupture that could be responsible for the wall motion abnormality.
• New ECG abnormalities.
• Absence of other causes of transient LV dysfunction (such as acute myocarditis, pheochromocytoma, or intracranial bleeding).
DIFFERENTIAL DIAGNOSIS
• The differential diagnosis includes any process that may cause acute, reversible LV dysfunction. Conditions to consider include ischemic heart disease, myocarditis of any etiology, coronary vasospasm/Prinzmetal’s angina, pheochromocytoma, intracranial bleeding/trauma, afterload excess states, cocaine abuse, and cardiac syndrome X.
MANAGEMENT
The most important aspect of management is recognition of this syndrome and appreciation of its often transient nature. For most patients, therapy is both supportive and expectant. At present, there exists no randomized or controlled clinical trial data to define the optimal management strategy.
• As these patients present with LV systolic dysfunction, they often exhibit heart failure symptoms. Treatment with ACE-inhibitors or ARBs, β-blockers, and diuretics is indicated.
• For patients with LV or RV thrombus observed on imaging studies, warfarin anticoagulation is recommended for a period of time until the wall motion abnormalities have resolved. Some investigators recommend anticoagulation for extensive LV apical wall motion abnormality independent of the presence of thrombus seen on an imaging study.
• For patients with severely compromised hemodynamic status, LV outflow tract obstruction (present in 10%-15% of such cases) should be excluded by echocardiography.
![]() For those without obstruction, inotropic support is indicated; an IABP may be required in select cases.
For those without obstruction, inotropic support is indicated; an IABP may be required in select cases.
![]() For those with documented LV outflow tract obstruction, inotropic agents should be withdrawn, and gentle administration of fluids along with cautious use of β-blockers is suggested; refractory hypotension may require support with a pure α-agonist such as phenylephrine.
For those with documented LV outflow tract obstruction, inotropic agents should be withdrawn, and gentle administration of fluids along with cautious use of β-blockers is suggested; refractory hypotension may require support with a pure α-agonist such as phenylephrine.
FOLLOW-UP
Stress-induced cardiomyopathy is a most often a transient disorder, and the vast majority of patients make a prompt, full recovery usually within 1 to 4 weeks (Figure 5-4-4).
• A minority of patients (about 5%) show delayed recovery of LV function over a period of 2.5 to 12 months.6
• Controversial at present is whether or not to continue with ACE-inhibitors and/or β-blockers once LV function has recovered fully.
• In a retrospective study with a mean follow-up of 4.4 years, 11.4% of patients experienced a recurrence within 4 years.9 Survival in this study was not different to compared to an age- and gender-matched population.
• Another group of investigators has reported that survival was reduced in comparison with an age- and gender-matched population; however, the excess mortality was due to noncardiac diseases and often occurred within the first year following the index hospitalization. A second study6 reported a recurrence rate of 5%.
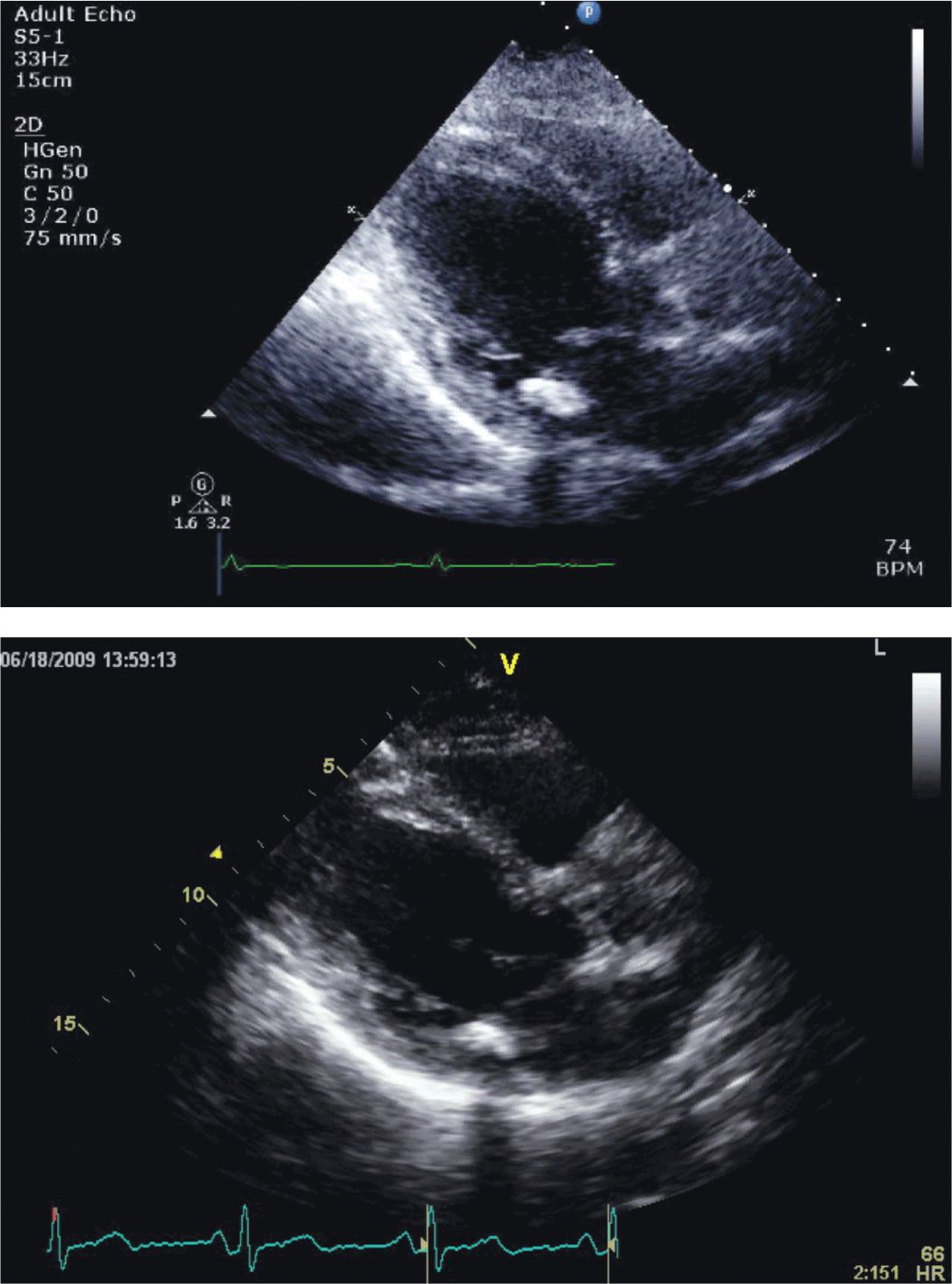
FIGURE 5-4-4 Parasternal long-axis images of the patient described in the case vignette. The image obtained on presentation (top panel) shows hyperkinetic contraction of the basal portions of the septum and inferolateral walls with akinesis of the remaining segments. Note involvement of the right ventricular free wall. In a follow-up image obtained 10 days later (bottom panel), LV systolic function has returned to normal.
REFERENCES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


