Fig. 20.1
Radial ultrasound miniprobe (20 MHz UM-S20-17S, Olympus Inc, Tokyo, Japan) introduced via a guide sheath through the working channel of a flexible bronchoscope. On the right, guide sheath only without miniprobe (Reprinted with permission from Olympus Europe Holding GmbH, Hamburg, Germany)
Ultrasound Imaging of Peripheral Lung Lesions
The most commonly used frequency in radial EBUS is 20 MHz, which provides a high resolution and allows detailed imaging of the structure of peripheral lung lesions. Normal air-filled alveolar tissue typically produces a “snowstorm-like” whitish ultrasound image due to differences in impedance (Fig. 20.2). Structures, which are close to the probe but separated by air, are not visible due to the total reflection of the ultrasound waves. If it is possible to reach a peripheral lung lesion with the probe, the ultrasonic picture will change. Solid tumours are usually well differentiated against the lung tissue by a bright border. They appear grey and more homogeneous in the ultrasound image (Fig. 20.3), although necrotic areas and vessels can be seen as circumscribed black areas. Furthermore, the existence of a continuous hyperechoic margin and absence of a linear-discrete air bronchogram indicate a suspicion for cancer. In contrast, the ultrasound images of inflammatory tissue or atelectasis have an inhomogeneous distribution, caused by the different structures of the lung. Small bronchi containing trapped air are visible as sharp white echo spots, fluid-filled areas also appear dark and borders are slightly blurred (Fig. 20.3).
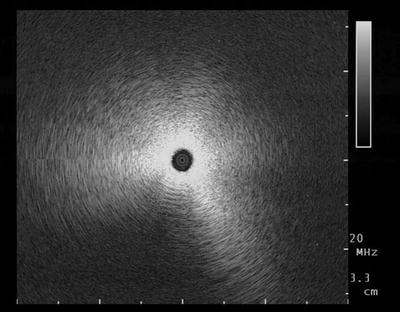
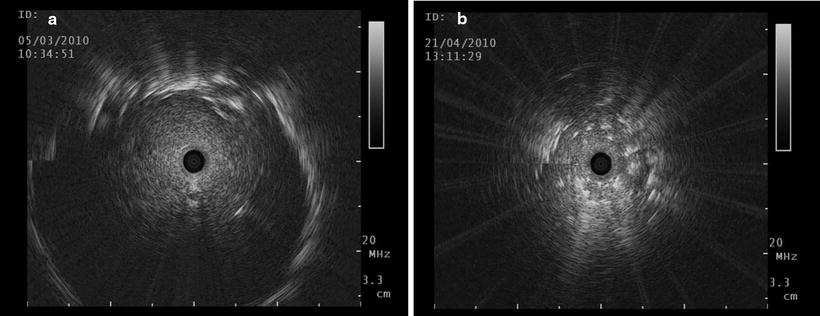

Fig. 20.2
A “snowstorm-like” ultrasound image, characteristic of normal ventilated, peripheral lung

Fig. 20.3
(a) Typical ultrasound image of a malignant solid tumour with clear borders. No air bronchogram is seen, which is a distinguishing sign for inflammatory disease or atelectasis; (b) white spots are visible centrally and the borders of the lesion are blurred in this benign lesion
Imaging Artefacts
The use of EBUS in the peripheral lung is simple, but interpretation of ultrasound images can sometimes be difficult. Fluids appear dark in the ultrasound images, but no Doppler mode is available in the radial ultrasound to identify vessels. To differentiate between necrotic areas and vessels, it is possible to follow the length of the lesion or to look for arterial vascular pulsation concurrent with the patient’s heartbeat. Trapped air shows sharp white spots with a “comet tail” sign behind, which looks similar to calcifications.
Further artefacts are possible, and this can lead to false interpretations. The radial image configuration starts at 9 o’clock, so during very quick movements, motion artefacts can be seen there as a sharp line. Strong reflections will create repeating echoes, which can be recognised by their consistent interval distance (Fig. 20.4).
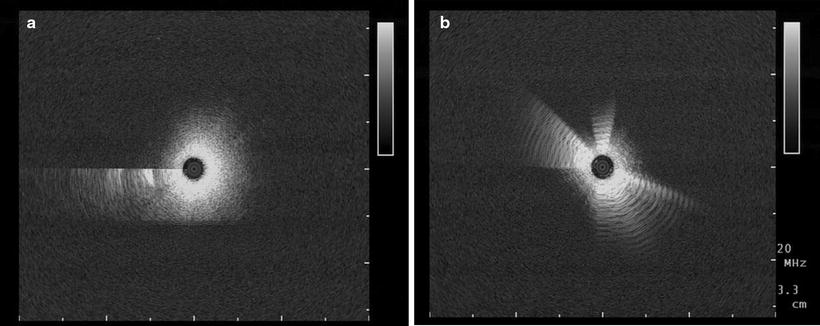

Fig. 20.4
Two specific artefacts which can be observed frequently: (a) a motion artefact at 9 o’clock and (b) repeating echoes surrounding the miniprobe
Technique of EBUS Navigation
The EBUS probes have to be advanced like a forceps into the different bronchi, where the lesion is suspected. This can be difficult in the apical segments and has to be performed carefully, because flexion and friction of the probe can damage the transducer and the connecting driving wire. A water-filled balloon is normally not necessary to provide sufficient contact to the surrounding structures when using the radial EBUS in the peripheral airways.
When solid round- or oval-shaped structures are visible, it indicates that the lesion is reached, and the probe is considered to be located within or adjacent to the lesion. After detection of the target, the probes must be removed from the working channel to introduce a biopsy tool for sampling specimens of the area of interest (Fig. 20.1).
Biopsy Tools
Transbronchial forceps biopsy is still the gold standard for sampling specimens from peripheral lung lesions. The tissue biopsy allows both histological and immunohistological examinations. In addition to the TBB, established biopsy tools for diagnosing peripheral lung lesions endoscopically are aspiration, brush sampling or transbronchial needle aspiration (TBNA). It has been shown that the yield will increase, when a cytological sampling is added to the TBB by forceps. Especially the sensitivity of TBNA in diagnosing peripheral lung lesions is greater than that of TBB in all papers in which these two techniques have been compared. In contrast, bronchial washing provides no added benefit to the diagnostic yield in patients undergoing bronchial brushing or TBB.
This applies to conventional bronchoscopy as well as to EBUS guidance. Concerning the different techniques, EBUS-guided TBNA showed the highest diagnostic yield compared with EBUS-guided TBB and bronchial washing. In a retrospective analysis of 155 patients, the yield for TBB in diagnosing SPN was higher, when the probe could be placed within the lesion (83%) compared to SPN in which the probe could only be placed adjacent to the target. No significant differences could be seen in the diagnostic yield for factors such as location, bronchus sign seen on CT, underlying disease, operator and type of EBUS probe used (Fig. 20.5).
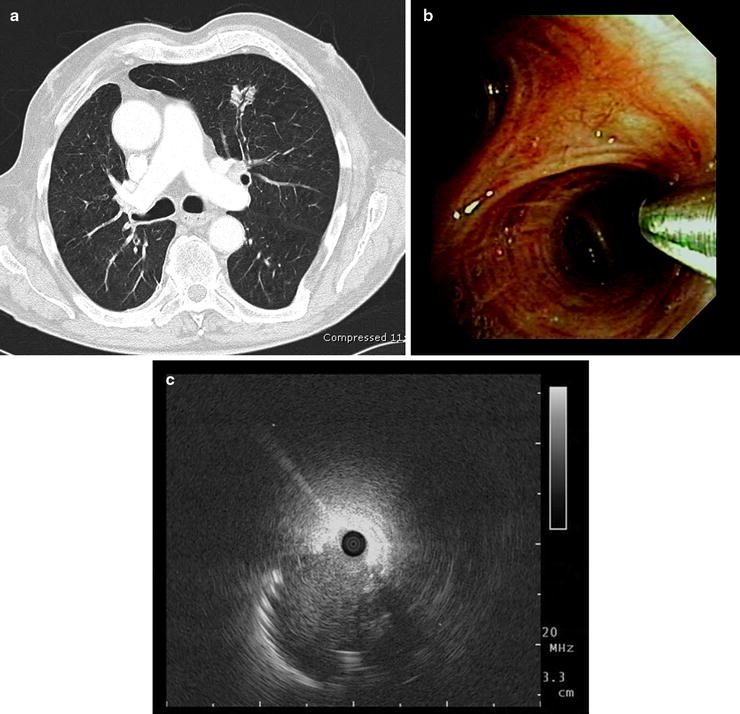

Fig. 20.5
(a) Bronchus sign: a bronchus leads directly to the peripheral nodule in the left upper lobe; (b, c) after introducing the miniprobe under endoscopic control in the anterior segmental bronchus, the lesion could be detected. Although the ultrasound probe could be placed only adjacent to the lesion, a non-small cell lung cancer was diagnosed with transbronchial forceps biopsy under fluoroscopic control (Part (a) courtesy of Prof. Dr. Claus P Heußel, Department of Diagnostic and Interventional Radiology, Thoraxklinik at the University of Heidelberg)
Applying TBNA to EBUS-guided bronchoscopy can further increase the diagnostic yield of SPNs without additional risk. The diagnostic advantage of TBNA becomes more obvious if the EBUS probe is only adjacent to the lesions. For malignant lesions, sampling by catheter aspiration was also associated with a higher diagnostic yield than sampling by forceps biopsy alone, in particular when EBUS could not confirm lesion location prior to sampling.
The disadvantages of cytological assessments are the requirement for an experienced cytopathologist and the difficulties in obtaining samples sufficient for immunochemistry.
Flexible cryoprobes which can be used for transbronchial histological sampling through a guide sheath may overcome these problems. After EBUS guidance, big biopsies without crush artefacts can be taken with this new approach, apparently without increased risk for pneumothorax and/or bleeding (Fig. 20.6).
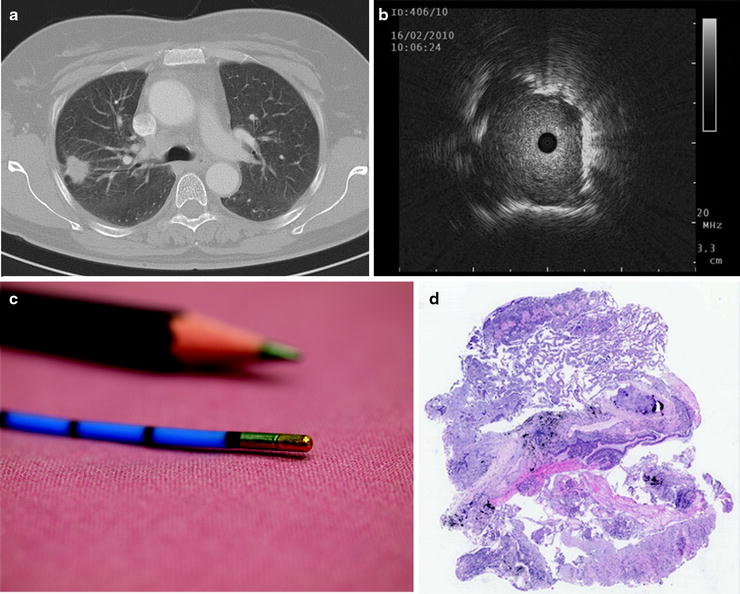

Fig. 20.6
(a) CT scan of a 50-year-old female with a solitary pulmonary nodule in the right upper lobe. (b, c) The lesion was detected with an EBUS miniprobe, and a cryobiopsy was taken with a flexible cryoprobe (no. 120416-037, 1.9-mm external diameter, length 900 mm, Erbe Medizintechnik, Tübingen, Germany) via guide sheath. (d) The pathologist estimated a large biopsy of excellent quality without any crush artefacts (HE staining). The final diagnosis established by EBUS-guided cryobiopsy was a micropapillary adenocarcinoma (Part (a) courtesy of Prof. Dr. Claus P Heußel, Department of Diagnostic and Interventional Radiology, Thoraxklinik at the University of Heidelberg; part (c) reprinted with kind permission of Horst Bryant, Heidelberg, Germany; part (d) courtesy of Prof. Dr. Philipp A. Schnabel, Department of Pathology, University of Heidelberg)
Fluoroscopic Guidance
In 2002, a report on the use of radial EBUS for SPN was first published. This was a prospective study comparing the diagnostic yield of fluoroscopy-guided versus EBUS-guided TBB, and diagnostic material was obtained in 76% with fluoroscopy versus 80% with EBUS guidance. A non-significant trend was observed towards EBUS being better than fluoroscopy for lesions of less than 3 cm in diameter.
Today, the most common additional approach for reaching peripheral lesions with EBUS is the use of fluoroscopy, which aids in directing the EBUS probe towards the peripheral lesion. Once the lesion has been detected in the ultrasound image and confirmed by fluoroscopy, a forceps is then introduced via the working channel of the bronchoscope and directed into the subsegmental bronchus. This is again controlled by fluoroscopy, and TBB can now be taken under fluoroscopic guidance. Even though the EBUS probe has to be removed prior to taking the TBB and this is therefore not a real-time procedure, a high yield can be achieved this way. Another advantage is the possibility to use a bigger forceps under guidance in order to obtain larger biopsy samples.
EBUS via Guide Sheath
Kurimoto first introduced the use of endobronchial ultrasound together with a guide sheath. For this technique, the bronchoscope is first advanced into the target bronchus under direct vision, and then the EBUS probe within a guide sheath is introduced via the working channel of the bronchoscope. A standard bronchoscope with a working channel of 2.0-mm diameter as well as a catheter with diameter of 1.9 mm can be used for the 1.4-mm probe. For the larger ultrasound probes of 1.7 mm, a bronchoscope with a working channel of 2.8 mm or larger and a guide sheath of 2.7 mm should be used. Now EBUS imaging confirms that the probe within the guide sheath has reached the lesion, and the EBUS probe is removed leaving the guide sheath in place. This guide sheath can now be used as an extended working channel in order to sample the lesion with either forceps, brush or curette. It is possible to use additional imaging with fluoroscopy to obtain the specimens. A retrospective analysis showed the optimum number of biopsy specimens with ultrasound guidance to be at least five.
In cases where the SPN cannot be visualised by EBUS, the probe should be removed from the guide sheath and a double-hinged curette can be inserted. This curette can now be manipulated under fluoroscopic guidance to identify the appropriate bronchus. Once it has been found, the curette is removed again and the EBUS probe is advanced to confirm the correct position on ultrasonographic picture (Fig. 20.7).
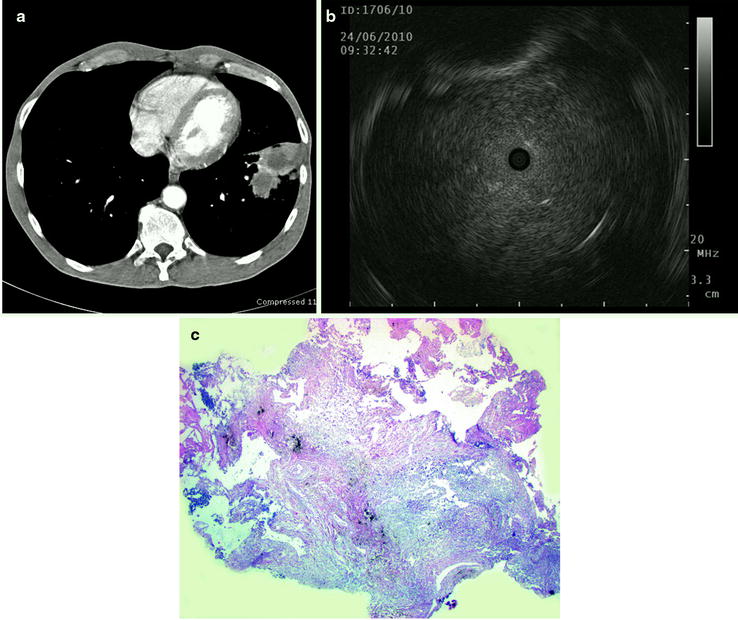

Fig. 20.7
(a) A 58-year-old male with a pulmonary lesion in the left lower lobe, (b) corresponding ultrasound image with homogeneous structure and clear margins suspicious of malignancy, (c) histological cross section of transbronchial forceps biopsy shows necrotic inflammation. The diagnosis of lung abscess was confirmed by surgical resection of the lesion (Part (a) courtesy of Prof. Dr. Claus P Heußel, Department of Diagnostic and Interventional Radiology, Thoraxklinik at the University of Heidelberg; part (d) courtesy of Prof. Dr. Philipp A. Schnabel, Department of Pathology, University of Heidelberg)
EBUS in Diagnosing Small or Non-visible Peripheral Pulmonary Lesions
Nodules less than 3 cm frequently cannot be visualised fluoroscopically. One prospective study assessed the diagnostic yield of EBUS-guided TBB in fluoroscopically invisible SPN and was able to show that in 80% the lesion was localised with EBUS (mean diameter of 2.2 cm) and a diagnosis was established by biopsy in 70%. EBUS can hence be used as an alternative to fluoroscopy in providing image guidance for TBB. Yoshikawa could further confirm the usefulness of endobronchial ultrasonography as a guide for diagnosing peripheral pulmonary lesions without the use of radiographic fluoroscopy. Seventy-six of 123 SPN (61.8%) were diagnosed by EBUS-guide sheath alone. In this study, the diagnostic yield for lesions > 20 mm was significantly higher than for those ≤ 20 mm in diameter. In a prospective study, 100 patients with SPN less than 20 mm were assessed with the guide sheath technique. Although the diagnosis was established only in 46 patients (46%) by EBUS-guided TBB, for those lesions which were detected by endobronchial ultrasound, the diagnostic success was 69%, which is similar to other studies. Therefore, ultrasound guidance may be more successful than fluoroscopic guidance in sampling non-visible or small peripheral lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


