Figure 30–1.
Different features of J wave elevation as notching (a) or slurring (b) in inferior or lateral leads in patients with VF
Prevalence
The prevalence of ER in general population varies from under 1 to 13 %, depending on the age (predominant in young adults), the race (highest amongst black population), and the cut-off value for significant J point elevation (0.05 mV vs. 0.1 mV) [14, 16–19, 22, 23]. Using the same ECG criteria as reported by Haissaguerre et al., Tikkanen et al. reported the prevalence of ER as 5.8 % in a middle-aged population of 10,864 Finnish people. When we consider J point elevation ≥0.2 mV, the prevalence dropped to 0.33 % (0.7 % in the control group studied by Haissaguerre et al. [14]).
Pathophysiology of Early Repolarization (Mechanism)
Insights from Experimental Studies
The exact mechanism for ER is still unknown. In 1991, Antzelevitch and colleagues first proposed that transmural differences in early phases of cardiac action potential (phases 1 and 2) are probably responsible for inscription of the electrocardiographic J wave [24]. Subsequently, they obtained direct evidence in support of this hypothesis in arterially perfused canine ventricular wedge preparations in 1996 [25]. Briefly, arrhythmogenic platform is created by disporportionate amplification of repolarizing current in the epicardial myocardium due to a decrease in inward sodium or calcium channel currents or an increase in outward potassium currents mediated by Ito, IK-ATP, IK-Ach channels. The trigger and substrate for development of phase 2 reentry and VT/VF eventually emerge from the transmural dispersion in the duration of cardiac action potentials.
Insights from Genetic Testing
Because, ER was not associated with increased risk of SCD until recently, the genetic markers differentiating benign and arrhythmic forms of ER have not been identified. The importance of the genetic background in ER has recently been suggested by Haïssaguerre et al. when they showed that 16 % of cases with VF and ER have a family history of SCD [14].
Given the high frequency of the genetic background underlying the early repolarization pattern in the population, it is probably polygenic and influenced by environmental factors. One could hypothesize that common variants contribute to the ECG pattern of ER and that a combination of such variants or the cosegregation of common and rare variants leads to the malignant form of ER. Large multicentric studies using state-of-the-art genomics approaches on large cohorts with malignant forms of ER patients should lead to the identification of the underlying molecular bases in this lethal arrhythmia.
As described previously, rare monogenic forms of ER have been reported using a candidate gene approach. ER on ECG suggests a shift in transmural voltage gradient between epicardium and endocardium as a causal mechanism. An increase in Ito, IKr, IKs, IKACH, IKATP current or a decrease of sodium INa and/or calcium ICaL current could lead to this phenomenon.
Following these hypotheses, a candidate gene approach on 156 probands allowed the identification of a rare variant in KCNJ8, responsible for the pore-forming subunit of the IKATP channel, in a 14 year old girl who was resuscitated following an episode of sudden death due to VF with early repolarization syndrome [26]. Her coronary angiogram with ergonovine injection, MRI, and flecainide and isoproterenol challenge tests were normal. She experienced >100 episodes of recurrent VF unresponsive to beta-blockers, lidocaine/mexiletine, verapamil, and amiodarone. Recurrences of VF were associated with massive accentuation of the early repolarization pattern at times mimicking acute myocardial ischemia. Coronary angiography during an episode with 1.2 mVJ/ST elevation was normal. Isoproterenol infusion acutely suppressed electrical storms, while quinidine eliminated all recurrences of VF and restored a normal ECG which has persisted over a follow-up of 65 months. The precise pathophysiological mechanism is still been studied using in vitro reexpression of the mutant channel. Implication of KCNJ8 as a novel J-wave syndrome susceptibility gene has been confirmed by recent work of Medeiros-Domingo et al. [27]. They have search for KCNJ8 mutation in 101 unrelated subjects with (87 BrS phenotype and 14 ER pattern). Six hundred healthy individuals were also examined to assess the allelic frequency for all variants detected. One BrS case and one ERS case hosted the identical missense mutation S422L, which was reported previously [26]. KCNJ8-S422L involves a highly conserved residue and was absent in 1,200 reference alleles. This mutation seems to lead to a marked gain of function in the cardiac K(ATP) Kir6.1 channel and might represent a novel pathogenic mechanism for the phenotypic expression of both BrS and ERS.
Burashnikov and colleagues identified a missense variant in the β(beta)2 subunit of the cardiac L-type calcium channel in patients with early repolarization syndrome. Expression studies for this variant are not available as yet [28].
In parallel familial studies, several large pedigrees with malignant ER forms with autosomal dominant pattern of inheritance have been identified. In each of these families the prevalence rates of SCD and ER are higher than in the general population. A strong genetic background has been suspected in a large pedigree of 66 members with 11 (16.6 %) SCDs and two individuals with ER pattern (Fig. 30.2). Seven out of 11 SCDs occurred in individuals less than 35 years of age. Also, 11 (16.6 %) asymptomatic individuals have an early repolarization pattern. Furthermore, all the disease transmitters were identified to have variable expression of ER on ECG (slight notch at the end of the QRS). An ECG showing ER and VF before sudden death is available only for patient V-17. In a second pedigree, individuals across three generations suffered sudden cardiac death and five out of eight siblings have early repolarization pattern (data not shown). Classical genetic linkage analysis assuming an autosomal dominant form of inheritance of ER should allow identifying the disease-causing gene.
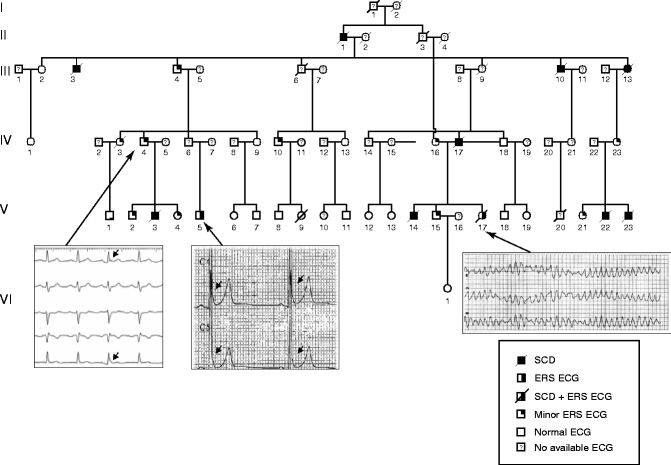

Figure 30–2.
Pedigree chart of 66 members with family history of electrocardiographic early repolarization syndrome (ERS ECG) and sudden cardiac death (SCD)
These data suggest an association between ER and SCD not only at a population level but also at a familial level demonstrating that early repolarization ECG pattern could be considered as a malignant syndrome associated with a high risk of SCD in some families.
Using two large, population-based cohorts (Framingham Heart Study (FHS) and Health 2000 Survey (H2K)), Noseworthy et al. have found higher prevalence of ER pattern among siblings (recurrence risk ratio of 1.89) suggesting a heritable basis of ER pattern in the general population [29]. In another large family-based cohort (1,877 individuals from 505 white nuclear families representative of the British general population), Reinhard et al. have found that offspring of parents with ER have a 2.5-fold increased risk of presenting with ER on their ECG [30].
Altogether these results strongly suggest that ER is a heritable phenotype. The familial pattern undermines the need for systematic familial screening for the identification of ER to limit the risk of sudden death within the family.
Early Repolarization or Delayed Depolarization: A Controversy on the Origin of J Wave
Although the J wave is synonymously used with early repolarization abnormality, the mechanistic evidence elucidating the inscription of J wave on surface ECG is incomplete. Basic investigators propose the inscription of J wave as coincident with phase 1 of cardiac action potential in the epicardial region of the ventricular myocardium which precedes phase 1 in the endo- and mid-myocardial cells generating an early gradient in the repolarization currents within the ventricles, thereby justifying J wave as an early repolarization phenomenon [24, 25]. In accordance with it, some clinical investigators concluded that J wave should be considered as a repolarization phenomenon rather than late depolarization because of its slower inscription, spontaneous/rate dependant fluctuation in morphologic pattern (increased pattern at slow heart rate, decreased pattern at faster heart rate) or amplitude in the face of stable QRS complexes, and amplitude varying concurrently with ST segment. These investigators did not find late potentials on high-amplification electrocardiography and invasive endocardial mapping further reinforcing their view [14].
In a recent work on deciphering the pathophysiology of J wave in ER syndrome, 22 idiopathic VF patients were monitored for 24 h using a newly developed signal-averaging system to record late potentials (depolarization marker), T-wave alternans and QT dispersion (repolarization markers) [31]. Frequency-domain heart rate variability (HRV), which reflects autonomic modulation, also was assessed. The incidence of late potentials in 7/22 (32 %) patients with VF and ER was higher than in the remaining 15 VF patients without ER (86 % vs. 27 %, P = .02). In contrast, repolarization markers did not differ between the two groups. Moreover, dynamic changes in late potential parameters (fQRS, RMS40, LAS40) were observed and were pronounced at night time only in the patients with VF and ER and high-frequency components (vagal tone index) on frequency-domain HRV analysis were associated with J waves in VF patients (P < .05). These investigators concluded that since idiopathic VF patients with ER had a high incidence of late potentials showing circadian variation with night ascendancy, J waves due to ER may be more closely associated with depolarization abnormality and autonomic modulation than with repolarization abnormality. Recently, Roten et al. have studied the effect of Ajmaline on J wave in three groups of patients: 31 with Early Repolarization (symptomatic and asymptomatic); 21 with type 1 Brugada syndrome and 22 controls [32]. They showed that Ajmaline significantly decreased mean J wave amplitude in ER group from 0.2 ± 0.15 mV at baseline to 0.08 ± 0.09 mV (p < 0.001). QRS width prolonged significantly in all three groups, but prolongation was significantly less in ER group (+21 ms) compared to Br group (+36 ms; p < 0.001) or controls (+28 ms; p = 0.010). They conclude that these results indicate different pathogenesis for ER and BrS. The altered terminal QRS vector probably is responsible for the decrease in J wave amplitude in ER, although a specific effect of ajmaline on J waves cannot be excluded.
Relationship Between J Wave Elevation and Ventricular Fibrillation
Amplitude of J Wave
Amplitude of J wave is more important in VF patients compared to controls (2.15 ± 1.2 mm in IVF vs. 1.05 ± 0.2 mm) [14]. Tikkanen et al. reported higher relative risk of cardiac death with a J point elevation of 0.2 mV (RR-3.03 – CI:1.88–4.90, p = 0.001) compared to 0.1 mV (RR-1.30 – CI:1.05–1.61, p = 0.02) [18].
Spontaneous Dynamicity
Since most of the VF episodes cannot be predicted clinically in patients with sporadic episodes of VF, it is difficult to get further insights into the role of ER in the mechanism of VF. However, some of the patients with VF experience electrical storm during hospitalization unraveling the dynamics of ER in VF arrhythmogenesis. Haïssaguerre et al. performed serial ECGs during electrical storm (including frequent ventricular ectopy and episodes of ventricular fibrillation) in 16 subjects and all patients showed consistent and marked increase in the amplitude of J wave during the period of storm when compared with baseline pattern (from 2.6 ± 1 mm to 4.1 ± 2 mm, P < 0.001) [33]. Besides spontaneous accentuation of the J wave amplitude preceding electrical storm, spontaneous beat to beat fluctuation in the morphologic pattern of ER was also observed [14]. Out of 11 patients with VF and ER reported by Nam et al., five patients experienced VF storm during their stay in intensive care unit. ECGs recorded within 30 min of the VF storm exhibited global appearance of J waves [17]. These dynamic ECG features of ER appeared spontaneously for a transient period around the VF storm revealing the presence of a functional “substrate” for arrhythmia. Derval et al. reported a high degree of spontaneous fluctuation of J wave in all cases, such that at least 1 ECG from the index hospitalization showed no evidence of significant ER in 58 % of patients (11 of 19 patients with ER and IVF; Fig. 30.3). These major fluctuations were not explained by heart rate fluctuation [23].
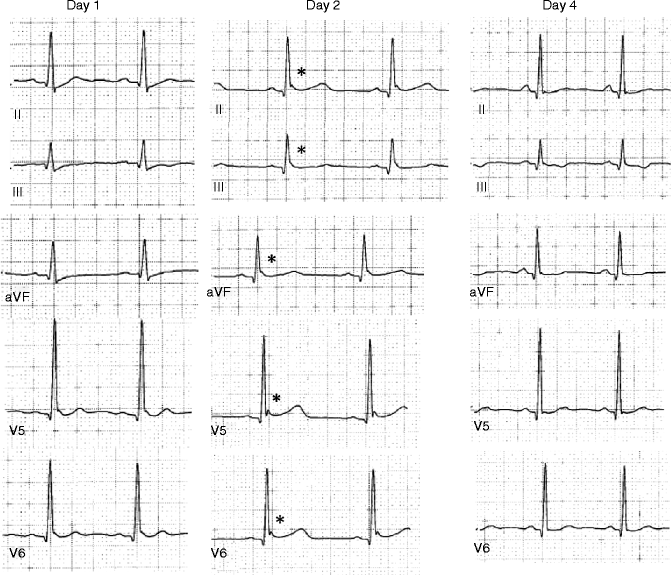

Figure 30–3.
Day-to-day fluctuation of ER pattern in an IVF patient. The ECG recorded at different times after cardiac arrest demonstrates fluctuation of J-wave amplitude (*) with typical Type I ER pattern (day 2), type II ER pattern (day 1) and no evidence of ER pattern day 4 after the cardiac arrest
Correlation Between J Point Location and Arrhythmia Origin
We mapped patients with ER and VF targeting the ventricular ectopy initiating the VF. In patients with ER recorded in inferior leads alone, all ectopies originated from the inferior left ventricular wall. In the subjects with widespread global ER, as recorded in both inferior and lateral leads, ectopy originated from multiple regions [14]. These findings prove that ER abnormality may be either limited to a single region in the ventricles or can extend beyond it to involve more than one region simultaneously. Whether J wave truly represents an abnormality of repolarization or not is still debated, but, these findings help towards localizing ER as an abnormality involving distal Purkinje tissue, its innervated myocardium or the Purkinje-myocardial junctions [34].
Electrocardiographic Phenotypes Associated with Favourable Outcome
Tikkanen et al. have collected and analysed ECG from large group of athletes (from Finland, n = 62; and US, n = 503) and in general population (middle aged, Finnish population; n = 10,864). ST segment after J wave were classified as horizontal/descending or rapidly ascending/upsloping [35]. Main findings were: (1) In athlete, ST segment in rapidly ascending/upsloping in the majority of cases; (2) Infero-lateral ER patterns are not associated with uniformly increased risk of arrhythmic death in a middle-aged general population. Subjects with ER patterns associated with horizontal/descending ST segment after J-wave had higher risk of sudden arrhythmic death (age- and sex adjusted HR 1.43; 95 % CI 1.05–1.94) than subjects without ER. Subjects with rapidly ascending/upsloping ST segment did not have an elevated risk for arrhythmic death (adjusted HR 0.89; 95 % CI 0.52–1.55). (3) No difference between prognostic significance of notching and slurring of ER patterns emerge from their study. They conclude that the highest risk of event occurred in patients with ER pattern combining: inferior location, high amplitude (above 0.2 mV), and a dominant horizontal or descending ST segment after J-wave.
Risk Stratification
As described above, although ER is a common entity, unexplained sudden cardiac arrest in young adults is very rare. Some investigators addressed this issue by using the Bayes’ law of conditional probabilities. Rosso et al. claimed that the presence of J-wave in a young adult would increase the probability of VF from 3.4:100,000 to 11:100,000 which is a negligible rise. They, therefore, concluded that the incidental discovery of J-wave on routine screening should not be interpreted as a marker of “high risk” for sudden death because the odds for this fatal disease would still be approximately 1:10,000 [16]. Now, the million dollar question is: “how to differentiate subjects with ‘high risk’ ER from the so-called benign ER?”
Clinical Features
In such a situation, we consider that close follow-up should be offered to the patients with ER and unexplained syncope or a family history of unexplained sudden death. Abe et al. reported that the prevalence of ER in 222 patients with syncope and no organic disorder was 18.5 %, which is almost ten times that in 3,915 healthy controls (2 %) [31]. Therefore, the possibility of ER-associated syncopal episodes cannot be excluded in at least some of these patients. The genetic basis of ER is still largely unknown. Also, in patients with VF and ER, positive family history of sudden death was not significantly higher than in those without ER (16 % vs. 9 %, P = 0.17) [14]. Nevertheless, it does not imply that family history is not an important aspect of history-taking in ER patients.
Magnitude of J Wave
In the study by Tikkanen et al., subjects with J-point elevation of more than 0.2 mV on inferior leads not only bore a higher risk of death from cardiac causes (adjusted relative risk, 2.98; 95 % CI, 1.85–4.92; P < 0.001) as compared with J point elevation of more than 0.1 mV, but also had a markedly elevated risk of death from arrhythmia (adjusted relative risk, 2.92; 95 % CI, 1.45–5.89; P = 0.01) [18]. This finding indicates that the magnitude of J-point elevation could be a discriminator of risk. However, this study did not provide the sensitivity and specificity of this measure in predicting the endpoint-events. In accordance with this finding, Haïssaguerre et al. also found that the magnitude of J wave elevation in case group was significantly higher than that in control subjects (2.0 ± 0.8 mV vs. 1.2 ± 0.4 mV, P < 0.001) [14]. Derval et al. also reported a higher magnitude of J-wave in patient with ER pattern and purely Idiopathic VF than in patient with ER pattern and another substrate for VF (0.25 ± 0.12 vs. 0.13 ± 0.05; p = 0.02) [23]. It is worthy to note that J point elevation of more than 0.2 mV seems rare in normal population. In 630/10,864 subjects with ER identified by Tikkanen et al., 0.33 % of total population had J wave elevation of more than 0.2 mV [18]. However, it is also necessary to point out that the magnitude of J wave elevation can fluctuate even without drug provocation or exercise. This means low magnitude of J wave should not be considered as a static entity. It can potentially get augmented. Unfortunately, there is no reliable provocation test to augment ER in infero-lateral leads currently.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


