Chapter 17 Dysfunctional breathing
INTRODUCTION
The term ‘dysfunctional breathing’ is, in itself, confusing. One would expect it to mean that a particular breathing pattern does not fulfil its function, yet it appears to be used as a synonym for hyperventilation. In a review Morgan (2002) remarks on ‘this new term’, that:
However, there is still no clear understanding of the relationships between the psychological, anatomical, physiological, pathological and behavioural variables as they appear to affect the central breathing pattern generator and/or disrupt the physical movements of breathing in differing ways.
CONTROL OF BREATHING
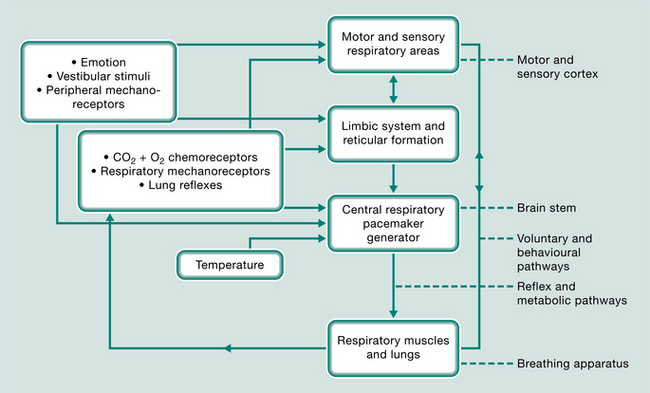
The respiratory system is the only autonomic function that can be controlled voluntarily. This complex system (Fig. 17.1) is self-sustaining and is centred on the respiratory-pacemaker-generator in the brain stem. It is moderated by oxygen and carbon dioxide chemoreceptors, lung tissue reflexes, mechanoreceptors in the ventilatory and the peripheral muscles and joints, emotion, temperature and vestibular influences. This sensory feedback may be filtered through, and integrated by, the reticular formation and limbic autonomic system or be transmitted directly to the breathing centre. Voluntary and behavioural stimuli from the cerebral cortex also feed back to the breathing centre and can influence the respiratory muscles directly by bypassing or overriding the reflex pathways. The cerebral cortex is also responsible for perceiving dyspnoea. Adaptation of any of the components of these motor or sensory pathways or centres of respiration could influence or concentrate disordered responses, breathing patterns and carbon dioxide levels. This results, in time, in what appears to be a resetting of the respiratory centre’s triggering mechanisms and the formation of new conditioned patterns of breathing.
Why are some people more prone to respond to physiological, psychological or environmental stimuli with grossly altered breathing patterns? Clark & Cochrane (1970), considering patients with chronic bronchitis and emphysema, concluded that the sensitivity of the respiratory centres is personality linked and related to mood-state and disposition. Papp et al (1993) discuss the theory of an inherently unstable autonomic nervous system and a hypersensitive respiratory control system. Cowley & Roy-Byrne (1987) suggest that this underlying biological and often inherited vulnerability, leading to a hypersensitive central nervous ‘alarm system’, may be triggered inappropriately, causing a premature ‘fight or flight’ response.
HYPERVENTILATION
Hyperventilation may be acute, it may coexist with organic disease or may occur in a chronic idiopathic form. In the past few years, together with ongoing research into the relationships between hyperventilation and panic disorders, researchers and practitioners have noted strong relationships between hyperventilation and vestibular and musculoskeletal disorders. These findings have now influenced some treatment programmes and investigative procedures.
Chronic hyperventilation
Clinically it can be recognized by the spontaneous occurrence of multiple and alarming symptoms.
In the table of their laboratory work they coined the term ‘hyperventilation syndrome’ (Kerr et al 1937). A syndrome is, by definition, identified by its combination of symptoms and is therefore not able to be contained within a single diagnostic measurement. The difficulty regarding the understanding of the various mechanisms producing the symptoms, of finding a definitive form of diagnostic test and agreeing a more appropriate name for this disorder, is still creating some discussion in the literature, as views change regarding the possible causes and effects (Gardner 2000, Hornsveld et al 1996, Howell 1997, Malmberg et al 2000, Troosters et al 1999). Howell (1997) and Tweeddale et al (1994) considered that ‘behavioural breathlessness’ may be a more appropriate term, but as many patients do not experience breathlessness as their main symptom (if at all) Howell considered at the time, that there was nothing to be gained by changing the name.
HYPERVENTILATION SYNDROME
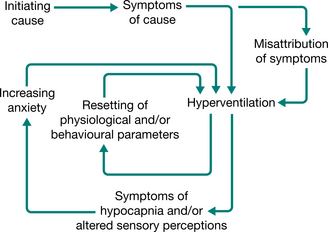
The diagnosis was not uncommon in the past (Baker 1934, Wood 1941) but in the present technological era, hyperventilation in its various chronic recurrent forms often tends to go unrecognized and the diverse symptoms are labelled as functional. Even though it has been shown that severe chronic hyperventilation with profound hypocapnia can be present in the absence of psychiatric, respiratory or other organic abnormalities (Bass & Gardner 1985), patients may attend a succession of clinics, presenting with increasingly disturbing symptoms and yet receive little help. The new anxiety aroused by the situation increases the hyperventilation–anxiety spiral, which may already be in operation. The spiral may be perpetuated by physiological and/or psychological causes, setting up conditioned reflexes of new and incorrect habitual patterns of breathing and a resetting, or loss of fine tuning, of the respiratory centre’s trigger mechanisms (Fig. 17.2).
Hyperventilation is today likely to be recognized more often in association with panic disorders or phobic states because of its causal, consequential or perpetuating relationships (Cowley & Roy-Byrne 1987). Patients presenting with panic disorder or anxiety (van den Hout et al 1992) or anxiety and evidence of depression (Jack et al 2003) have been shown to have lower resting levels of carbon dioxide than normal. Some may demonstrate a greater than normal sensitivity of the basilar artery’s response to hypocarbia (Ball & Shekhar 1997).
Hyperventilation has been shown to be prevalent in patients with vestibular dysfunction. This could be caused by the anxiety of the condition, but for some patients neither the hyperventilation nor the vestibular dysfunction provokes anxiety (Humphriss et al 2004). Ongoing work demonstrates a relationship between the vestibular and respiratory pathways in the brain stem, the contribution of the vestibular system to respiratory muscle activity and cardiovascular control during changes of posture and movement, and posture instability following hyperventilation (Yates & Bronstein 2005, Yates & Miller 1998, Yates et al 2002). Stress, acute pain and chronic pain may often result in hyperventilation producing hypocapnia (Terekhin & Forster 2006).
SIGNS AND SYMPTOMS
The vast array of commonly reported signs and symptoms can be loosely grouped as they affect different systems (Table 17.1). Timmons (1994) suggests that it would be useful to have a comprehensive validated list of symptoms, in order to hasten history taking and to facilitate comparisons of therapeutic trials. Few, if any, studies have looked at the association of symptoms with age, sex, personality traits and causal factors.
Table 17.1 Commonly reported signs and symptoms
| System | Signs and symptoms |
|---|---|
| Cardiovascular | |
| Gastrointestinal | |
| General | |
| Musculoskeletal | |
| Neurological | |
| Respiratory | |
| Psychological/psychiatric |
Some patients present with a constant resting level of hypocapnia and others present with resting levels of carbon dioxide within the normal range. Both groups feel generally unwell and experience ‘attacks’ featuring a galaxy of symptoms, which appear to occur sometimes with no apparent reason. Further hypocapnia may or may not be recorded at these times. In some patients the symptoms seem to precede the event of hypocapnia rather than be a consequence (Hornsveld et al 1996). If symptoms in some patients are not generated by hypocapnia, we must ask what other mechanisms could be the cause. Could the symptoms be a direct consequence of the disordered movements of overbreathing and their abnormal feedback? And if so, which sensory, motor or behavioural mechanisms could be involved?
Hypocapnia induces vascular constriction, resulting in decreased blood flow, and as a response to the Bohr effect there is also inhibition of transfer of oxygen from haemoglobin in the circulating blood to the tissue cells. Hypocapnic vasoconstriction is probably the cause of the characteristic range of cerebral, peripheral and cardiac symptoms. As the cerebral symptoms of hypoglycaemia are similar to those of hypocapnia, the cerebral effects of hyperventilation are highlighted at times of low blood sugar and it has been suggested that hyperventilation increases circulating histamine, which may be the cause of the high incidence of allergies reported (Lum 1994). Fluctuations in PaCO2 can have a destabilizing effect on the autonomic system resulting in a sympathetic dominance (Freeman & Nixon 1985). The patients are often in a state of arousal. It has been shown that the mean urinary excretion of adrenaline in a group of persons who hyperventilate was three times as high as in a group of normals (Folgering et al 1983). Mogyoros et al (1997) suggest that hyperventilation has a selective action on nerve conduction, which is greater on sensory than motor fibres. This may explain why hyperventilation induces paraesthesia before fasciculation.
Altered patterns of breathing can cause musculo-skeletal dysfunction. The combination of habitual overuse, ischaemia and increased sympathetic drive increases myofascial tone via smooth muscle cell contraction and leads to the evolution of active myofascial trigger points (Chaitow 2002). Pain thresholds are also reduced. Patients with dysfunctional breathing may describe a history of neck and/or thoracic pain and stiffness, headaches, low back pain and temporomandibular joint dysfunction. Orofacial pain is also associated with this patient group (Hruska 1997).
The diaphragm has a place in postural control and spinal stability, such that control of intra-abdominal pressure occurs through coordinated activity of the diaphragm, pelvic floor and transversus abdominis muscles (Hodges & Gandevia 2000, Hodges et al 1997). Dysfunction in this stabilizing mechanism has been implicated in recurrent low back pain. Postural activity in the diaphragm (and transversus abdominis) was shown to be reduced or absent after only 60 seconds of experimentally induced hypercapnia, suggesting that stability of the spine may be compromised in some respiratory disease or dysfunction (Hodges et al 2001). It is hypothesized that such a compromise may lead to increased potential for injury to spinal structures and reduced postural control (Hodges et al 2001); however, further research is indicated. Disorders of breathing (and continence) have been shown to have a higher association with low back pain than obesity and physical activity (Smith et al 2006).
Alternatively, acute or chronic musculoskeletal disorders may precipitate breathing dysfunction due to pain, postural compensations, and structural and functional changes in the fascial, neural, muscular and skeletal systems. The typical flexed posture of a person in pain impedes normal respiratory function. Musculo-skeletal dysfunction such as thoracic spine hypomobility, rib immobility, and short overactive accessory respiratory muscles can directly cause dysfunctional breathing patterns to develop (Chaitow 2002).
The relationship between chronic fatigue syndrome (CFS) and hypocapnia is questioned and Naschitz et al (2006) suggest that unrecognized hypocapnia is common in CFS. Interestingly, patients with CFS also frequently experience chronic musculoskeletal pain (Nijs et al 2006). Patients with hypermobility syndrome commonly present with dysfunctional breathing patterns and chronic pain. Hypermobility syndrome may have an effect on ventilation because of abnormally compliant lungs, disordered lung receptors, hypermobility of the thoracovertebral joints and/or pain. Hypermobility syndrome has been shown to be associated with fibromyalgia (Ofluoglu et al 2006) and CFS (Nijs et al 2006). Could dysfunctional breathing be the common link between these three conditions?
CAUSES OF HYPERVENTILATION
The many circumstances that may stimulate a hyperventilatory response (Box 17.1) may or may not become a chronic disorder. Other than HVS being the primary cause of the patient’s symptoms, we have seen that it is not uncommon for chronic hyperventilation to coexist with other conditions and to be a sustaining factor within the complex interaction of a number of physiological, organic and psychological disorders (Gardner 1994). Before embarking on a treatment programme, it is necessary to ensure that the patient has been suitably investigated in order to diagnose any underlying treatable disease or disorder and to recognize any other possible coexisting factors. Where pain is believed to be a causative or contributing factor, appropriate management strategies should be investigated.
Box 17.1 Some causes of hyperventilation
| Drugs | |
| Organic disorder | |
| Physiological | |
| Psychiatric | |
| Psychological |
DIAGNOSTIC TESTS
The voluntary hyperventilation provocation test (HVPT)
The hyperventilation provocation test (HVPT) (Hardonk & Beumer 1979) records end-tidal PACO2 and all symptoms provoked during and after the test. Using end-tidal PACO2 recordings, the patient is requested to hyperventilate for 3 minutes. If the PACO2 falls by at least 1.33 kPa (10 mmHg) and the level of recovery is less than two-thirds of the former resting level after 3 minutes, the result is recorded as a positive diag- nosis of a hyperventilation syndrome. Nevertheless, as about a quarter of ‘normals’ also show this phenomenon, a lowering of PACO2 and slow recovery is not in itself diagnostic. Immediately after the completion of the recordings the patient is asked to compare any symptoms provoked during the test with recognized complaints. When two or more major symptoms are reproduced, the HVPT is considered positive. Generally, tingling of fingers and dizziness are not included because these symptoms occur in ‘normal’ subjects. However, some patients report that some provoked symptoms are only ‘similar’ but not the ‘same’. Sometimes provoked symptoms are new experiences and not related to the patient’s complaints. Others may be helped by recognizing that altered breathing can precipitate their symptoms. Caution should be exercised on using this test if the patient complains of cardiac symptoms or pseudoangina. As yet this test has not been standardized.
The Nijmegen questionnaire
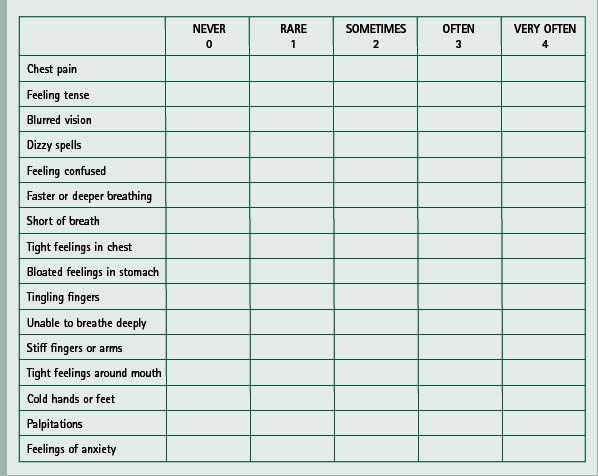
This was first drawn up as a list of 16 complaints chosen by a team of specialists from different disciplines, from 45 clinically relevant symptoms related to hyperventilation syndromes (van Doorn et al 1982). The complaints fall into three categories or dimensions, corresponding with the classic triad of breathing disruption, paraesthesiae and central nervous system effects. The list does not include fatigue or behavioural disturbances. Patients score on a five-point scale from 0–4 (0 = never, 1 = rare, 2 = sometimes, 3 = often, 4 = very often) against each of the 16 listed symptoms and a score over 23 is recognized as positive (Box 17.2). The efficacy of the questionnaire was investigated by comparing patients who hyperventilate with persons who do not. It showed a high ability to differentiate between the two groups (van Dixhoorn & Duivenvoorden 1985) and, although not conclusive, it was recognized that the questionnaire was suitable to be used as a screening instrument in diagnosing HVS when used with additional information. A correlation has been shown between positively rated Nijmegen questionnaire results (score of 24 or more) and positive HVPT results (recognition of at least two major symptoms) (Vansteenkiste et al 1991).
Humphriss et al (2004) found that, despite some limitations, the Nijmegen questionnaire is a quick and easy to administer assessment tool to detect HVS in patients seen for vestibular assessment. It is important to diagnose the condition in vestibular disease as it has been suggested that hyperventilation disrupts vestibular compensation and increases postural sway. Conventional vestibular rehabilitation may be only suboptimally successful until a normal breathing pattern has been re-established. The questionnaire has been used to diagnose HVS in some patients complaining of nasal congestion and subsequent breathing re-education has been successful in correcting the nasal congestion without further surgery (Bartley 2005).
The ‘think test’
The ‘think test’ (Nixon & Freeman 1988) provides a patient-specific stimulation, which can have an advantage over unspecific challenges in testing for episodic hypocapnia or a change in the breathing pattern. The patient is invited to close the eyes and then to recall the emotions and sensations surrounding certain situations that have caused distress or provoked symptoms. A change in the breathing pattern and/or a fall in PaCO2 greater than 1.33 kPa (10 mmHg) is considered to be significant.
Ambulatory monitoring of transcutaneous carbon dioxide
Prolonged monitoring may produce helpful data (Hibbert & Pilsbury 1988).
Patients with recurrent laryngeal nerve palsy can become fatigued during prolonged phonation, due to excessive breath loss while trying to maintain sufficient subglottal pressure for speech. Transcutaneous carbon dioxide (PTcCO2) recordings have shown significant decreases in PaCO2 in these patients. Education of the disrupted pattern and control of the expiratory phase during vocalization has produced improvement in PaCO2 levels on PTcCO2 and a decrease in symptoms (Miyazaki et al 1999).
Breath-holding time
This is a semi-objective measure that generally shows a direct relationship between the maximum breath-holding time (BHT) and the resting PaCO2 (short breath-holding time is usually associated with a low or unstable resting PaCO2). Breath-holding time tends to increase as the breathing pattern becomes more regular and stable. The patient is given a description of the method and then asked to take a normal breath in, followed by a normal breath out and to hold the breath in the resting phase until it becomes too uncomfortable to hold any longer. This breath-holding time is recorded. Some ask for the breath-hold after a full inspiration and a full expiration. Whichever method is used, it is essential to use a similar process and a similar posture each time. Standardization of the procedure would be helpful. The average BHT is suggested to be approximately 30 seconds (Bradley 2002). The patient will hope to increase the BHT if it is low but it is not helpful to know the norm at this time, as it may cause a forced hold time, which could be damaging to the process. If breath-holding time is recorded at regular intervals it could be used as a semi-objective outcome measure.
PATTERNS OF BREATHING
The respiratory rate and volume may be extremely irregular (Fig. 17.3) and the pattern interspersed with sighs, sniffs and yawns. At the other extreme, once habitual hypocapnia has been established, it may require only an occasional deep sigh to maintain the new low levels of carbon dioxide and the general breathing pattern may appear normal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree