Synucleinopathies
Lewy body disorders
Parkinson disease
Primary motor disease with parkinsonism and frequent peripheral autonomic neuropathy, orthostatic hypotension, and dementia
Dementia with Lewy bodies
Rapidly progressive dementia with frequent peripheral autonomic neuropathy, orthostatic hypotension, and parkinsonism
Pure autonomic failure (idiopathic orthostatic hypotension)
Sporadic, primary peripheral (postganglionic) autonomic neuropathy that occurs isolated from other neurologic disorders
Multiple system atrophy (MSA)
Sporadic, central (preganglionic) autonomic neuropathy with orthostatic hypotension, parkinsonism, and cerebellar ataxia in any combination
MSA–parkinsonian
Formerly designated striatonigral degeneration. The striatum and mesencephalon are especially vulnerable and dysfunctional, and parkinsonism dominates the clinical picture.
MSA–cerebellar
Formerly designated olivopontocerebellar atrophy. The brainstem and cerebellum are especially vulnerable and dysfunctional, and cerebellar features dominate the clinical picture.
Shy–Drager syndrome
Autonomic failure dominates the clinical picture.
Diabetes mellitus
Peripheral autonomic neuropathy with frequent orthostatic hypotension
Toxicity
Peripheral autonomic neuropathy caused by alcohol, chemotherapy drugs, metal poisoning, or ganglionic blockers
Amyloidosis
Autonomic neuropathy caused by neuronal amyloid protein deposition
Autoimmune ganglionic neuropathy
Peripheral autonomic neuropathy caused by antibody targeting of the α3 subunit of the acetylcholine receptor at the autonomic ganglia level
Familial dysautonomia (Riley–Day syndrome)
Autosomal recessive disease of the autonomic nervous system with loss of neuronal fibers (most commonly seen in Ashkenazi Jews)
Neurodegenerative Diseases
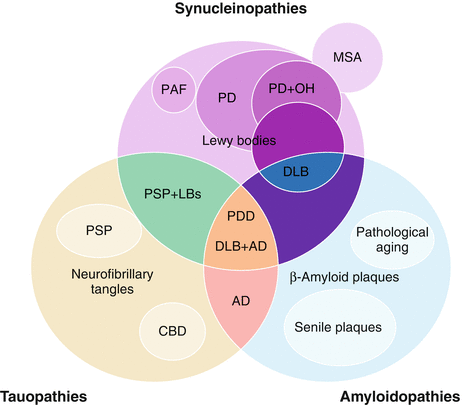
Figure 9-1.
Some of the most severe forms of dysautonomia appear in neurodegenerative diseases. Aggregation of misfolded protein is a common characteristic of many neurodegenerative disorders, with considerable interaction between pathologic/toxic proteins and neurodegeneration. Dysautonomia is common in the synucleinopathies, a group of disorders characterized by intracellular cytoplasmic inclusions of abnormal accumulation of misfolded α-synuclein protein. Synucleinopathies include Lewy body disorders (Parkinson disease [PD], dementia with Lewy bodies [DLB], and pure autonomic failure [PAF]), as well as multiple system atrophy (MSA). Lewy body disorders are characterized by Lewy bodies and Lewy neurite aggregates, which mainly consist of α-synuclein assembled into fibrillar inclusions that stain with periodic acid–Schiff and ubiquitin [2, 3]. In addition, most of these neurodegenerative diseases, as well as others such as some tauopathies (characterized by accumulation of tau protein as a major constituent of neurofibrillary tangles, e.g. corticobasal degeneration [CBD] and progressive supranuclear palsy [PSP]), present with parkinsonian movement disorders, and some also present with dementia. The pathologic interaction among different neurodegenerative processes also includes the amyloidopathies, which also may present with dementia (e.g., Alzheimer disease [AD]). LBs Lewy bodies, OH orthostatic hypotension, PDD Parkinson disease dementia.
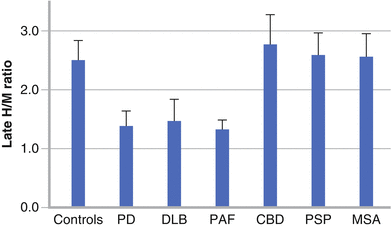
Figure 9-2.
The overlapping of pathophysiologic and clinical features among different neurodegenerative diseases often makes the differential diagnosis difficult, especially in the early stages or in patients with atypical symptoms, resulting in an initial misdiagnosis in nearly 25 % of patients with parkinsonism. Cardiac sympathetic imaging helps in this differential diagnosis, as can be seen here. The decreased cardiac 123I-MIBG uptake reflects the extensive distribution of Lewy aggregates through the nervous system (in both the central and peripheral nervous systems in PD and DLB, and in the peripheral nervous system in PAF), including the postganglionic sympathetic and intrinsic neurons in the heart (Adapted from Kashihara et al. [4]).
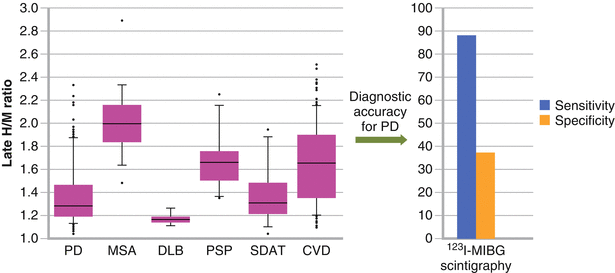
Figure 9-3.
In a study including 391 outpatients with parkinsonian symptoms and 10 healthy control individuals of similar age, 123I-MIBG was decreased in most patients with PD. However, more than half the patients without PD also exhibited low uptake, resulting in a sensitivity of 87.7 % and specificity of 37.4 % for detecting PD (H/M ratio cutoff value >2 standard deviations below the control mean), indicating that cardiac sympathetic denervation is not specific for PD. CVD cardiovascular disease, SDAT senile dementia of Alzheimer type (Adapted from Nagayama et al. [5]).
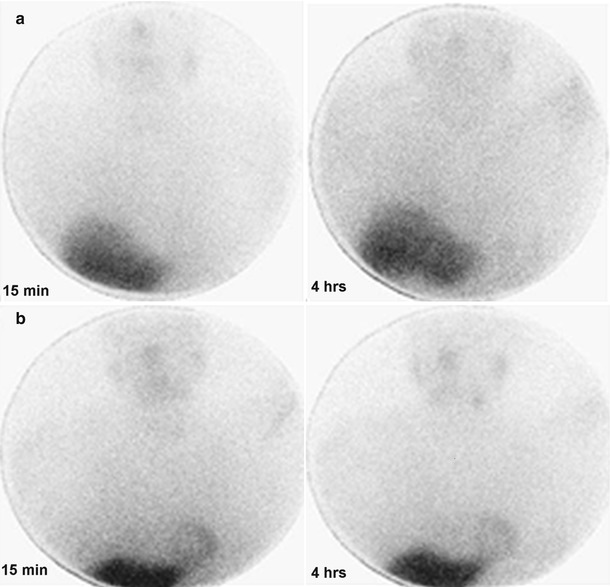
Figure 9-4.
Planar images of the thorax in the anterior view acquired at 15 minutes and 4 h after 123I-MIBG injection in a patient with PD (a) and a patient with MSA (b). Cardiac 123I-MIBG uptake is extremely reduced in the patient with PD, but only mildly reduced in the patient with MSA.
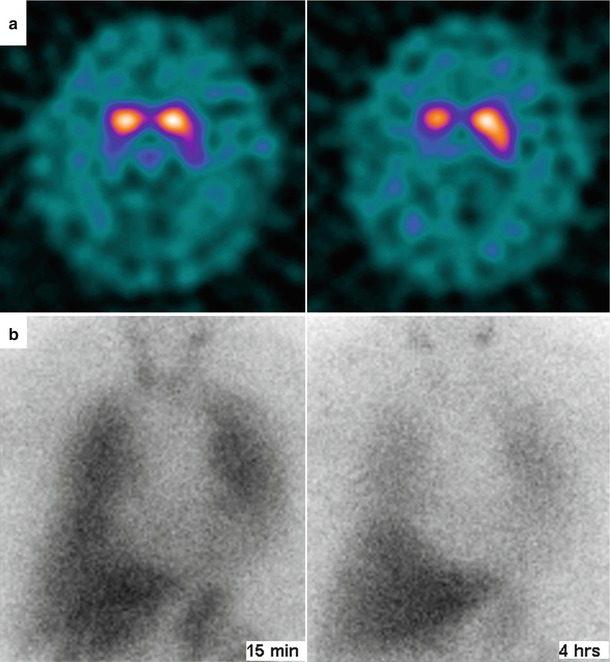
Figure 9-5.
Dopamine transporter (DAT) imaging by means of brain single-photon emission computed tomography (SPECT) with 123I- fluoropropyl (FP)-β-carbomethoxy-3 β-(4-iodophenyltropane) (CIT) usually is abnormal in patients with parkinsonian movement disorders, who show reduced striatal 123I-FP-CIT uptake. (a), This abnormality is seen in this patient with DLB, who also shows impairment of cardiac sympathetic innervation, as reflected by extremely reduced cardiac 123I-MIBG uptake. (b), Planar images of the thorax at 15 min and 4 h of 123I-MIBG injection.
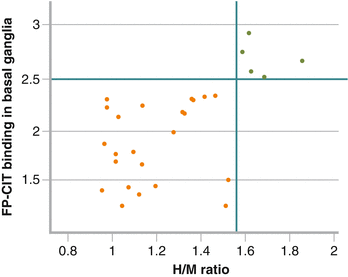
Figure 9-6.
In patients older than 65 years with cognitive decline, DLB is the second most common neurodegenerative dementia after AD, accounting for 10 % of all dementias [6–8]. Differential diagnosis between DLB and AD has important therapy implications, as DLB may benefit from treatment with cholinesterase inhibitors and carbidopa–levodopa, whereas it may worsen from treatment with anticholinergics and certain neuroleptic medications. Predominance of deficits in attention and visual perception over deterioration of memory and naming; psychiatric features, such as fluctuating cognition and recurrent visual hallucinations; parkinsonism; REM sleep behavior disorder; and dysautonomia are more common in DLB, but distinguishing DLB from AD is clinically challenging, especially in the early disease stages [6]. Among the different imaging techniques used in the diagnosis of DLB, DAT imaging has had the greatest effect so far. Exhibition of reduced DAT uptake in the basal ganglia is listed as one of the suggestive features of DLB [9]. However, cardiac 123I-MIBG imaging also has been advocated as useful in this setting because of the similarities between PD and DLB (Fig. 9.1) [10, 11]. Decreased cardiac 123I-MIBG uptake in DLB has been reported, whereas uptake is normal or near normal in AD. In a direct comparison between cardiac 123I-MIBG imaging and 123I-FP-CIT SPECT in the same cohort of DLB patients, Camacho et al. [12] found that all patients with reduced cardiac 123I-MIBG uptake (23 of 28 patients with a clinical diagnosis of DLB) also showed reduced striatal 123I-FP-CIT uptake (orange dots in the figure). Interestingly, the five patients with normal cardiac 123I-MIBG uptake (green dots in the figure) also showed normal striatal 123I-FP-CIT uptake. In addition, H/M ratio correlated positively with striatal 123I-FP-CIT uptake ratios (R = 0.6; P < 0.05). Horizontal and vertical lines in the figure reflect normal values for H/M ratio (1.56) and 123I-FP-CIT binding in basal ganglia (2.51). The severity of parkinsonism was highly correlated with striatal 123I-FP-CIT uptake but not with cardiac 123I-MIBG uptake. Other studies have shown that reduced cardiac 123I-MIBG uptake in DLB is not related to autonomic failure or parkinsonism [4, 11], but patients with DLB and orthostatic hypotension (OI) have lower 123I-MIBG uptake than patients with DLB without OI [13]. An advantage of cardiac 123I-MIBG over DAT imaging is the short acquisition time and comfortable planar imaging, which is appreciated by patients and their caregivers [14]. Current guidelines include abnormal cardiac 123I-MIBG imaging as one of the supportive features commonly present in DLB but not proven to have diagnostic specificity [9]. Debate is ongoing regarding whether abnormal cardiac 123I-MIBG scintigraphy should be upgraded in the guidelines as a feature suggestive of DLB, but evidence is still lacking and further studies are warranted [15] (Adapted from Camacho et al. [12]).
Cardiovascular Autonomic Diabetic Neuropathy
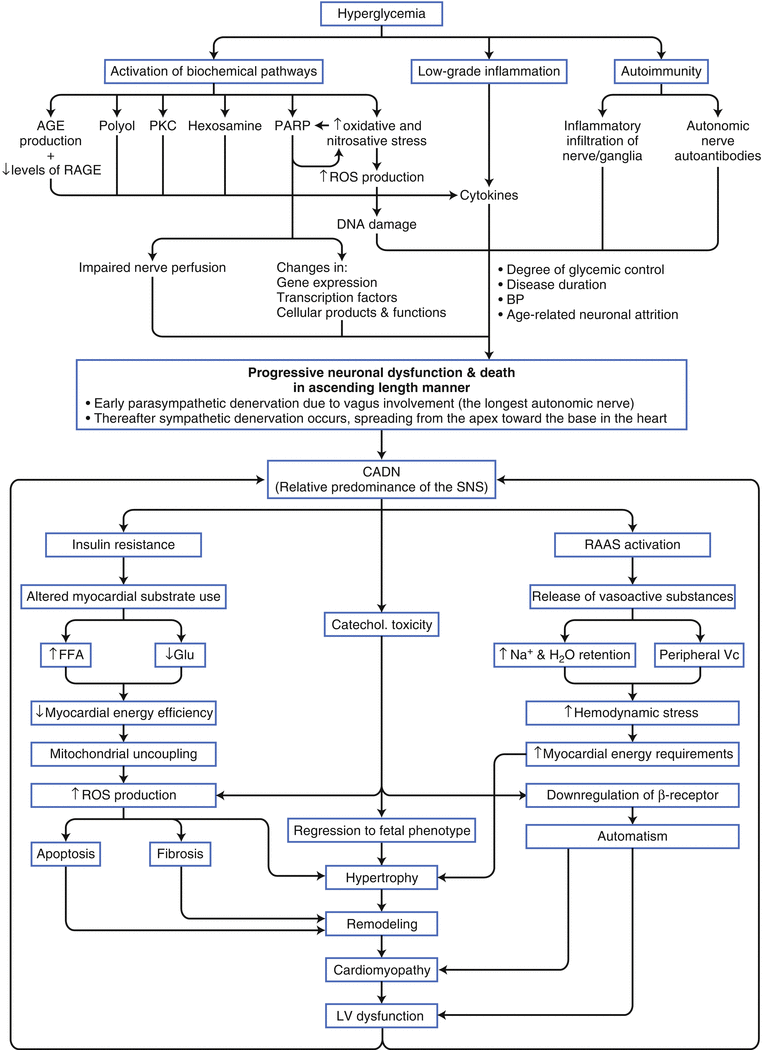
Figure 9-7.
Diabetes mellitus (DM) is the most common identifiable cause of neuropathy. Approximately 50 % of patients with type 1 (T1DM) or type 2 DM (T2DM) will develop diabetic neuropathy, which is a serious complication involving variable multiple organ involvement, principally of the cardiovascular system but also of the gastrointestinal and urogenital tracts. Cardiovascular autonomic diabetic neuropathy (CADN) is the primary disorder of diabetic neuropathy, and it is defined as the impairment of cardiovascular autonomic control in patients with established DM after the exclusion of other causes [16]. CADN likely has a multifactorial pathogenesis, as shown here, leading to neuronal ischemia and/or direct neuronal death or dysfunction, as well as left ventricular (LV) dysfunction. It probably is the most common form of autonomic dysfunction worldwide, and its prevalence varies between 1 and 90 % in patients with T1DM and 20 and 73 % in those with T2DM. Such wide variation is a result of the discrepancy in the criteria used to diagnose CADN and differences in the study populations in relation to CADN risk factors (conventional cardiovascular risk factors, glycemic control, and DM duration) [17]. AGE advanced glycation end products, BP blood pressure, Catechol catecholamine, FFA free fatty acids, Glu glucose, PARP poly (ADP-ribose) polymerase; PKC protein kinase C, RAAS renin–angiotensin–aldosterone system, RAGE receptor for advanced glycation end products, ROS reactive oxygen species, SNS sympathetic nervous system, Vc vasoconstriction.
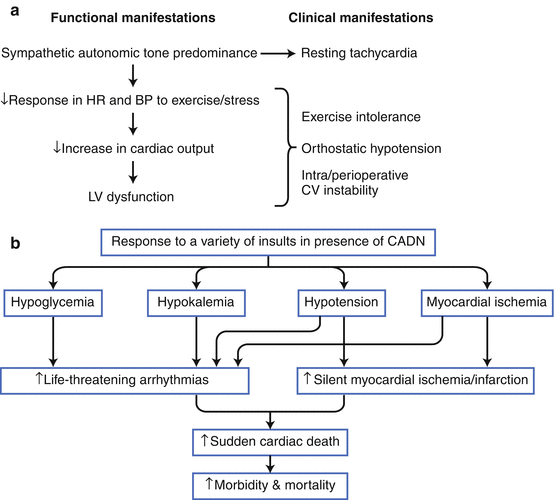
Figure 9-8.
(a), CADN may result in disabling clinical and functional manifestations, with an associated increased mortality risk. A meta-analysis showed that the pooled estimated relative mortality risk for DM patients with CADN was 2.14 (95 % CI, 1.83–2.51) [18]. In addition, among other risk factors, CADN has the strongest association with mortality [19]. (b), CADN remains a significant predictor of death after adjustment for conventional cardiovascular risk factors [20, 21], and CADN appears to be associated with major cardiovascular events [22]. Despite normal myocardial perfusion imaging in DM patients predicting an improved prognosis [23], the presence of CADN increases cardiovascular risk by mechanisms that are not fully understood [21]. CADN also is associated with a higher mortality risk in DM patients after a first myocardial infarction, which suggests that screening for CADN in these patients might be used for risk stratification [24].
Table 9-2.
Recommendations on screening for CADN in patients with DM.
T2DM at time of diagnosis; T1DM within 5 years of diagnosis, especially recommended in patients with other macro- and/or microvascular complications |
History of poor glycemic control |
Candidates to begin a new exercise program |
Perioperative assessment in patients with poor glycemic control and CAD |
After a first MI |
Table 9-3.
Cardiac autonomic reflex tests.
Standard CART (SCART): measures HR and BP response to certain physiologic maneuvers |
HR response to deep breathinga |
Assessment of beat-to-beat HR variation (R-R variation) during paced breathing (expiration-to-inspiration ratio [E:I]) |
HR response to standinga |
Expressed as the 30:15 ratio, which is the ratio of the longest R-R interval (between the 20th and 40th beats) to the shortest R-R interval (between beats 5 and 25) elicited by a change from horizontal to vertical position |
HR response to Valsalva maneuverb |
BP response to standing |
BP response to sustained hand grip |
HRV test: measures R-R interval |
RR interval frequency- or spectral-domain analysisc |
RR interval time- or statistical-domain analysis |
Baroreflex sensitivity (BRS) test: measures changes in HR in response to changes in (systolic) BPd |
Intravenous bolus injection of epinephrine/phenylephrine |
Physical maneuvers, such as postural change |
Criteria for diagnosis and staging of CADN |
Possible or early CADN: presence of a single abnormal CART result |
Definite or confirmed CADN: presence of two or three abnormal tests among the SCART and HRV tests |
Severe or advanced CADN: presence of orthostatic hypotension in addition to the criteria for definite or confirmed CADN |
123I-MIBG Imaging in CADN
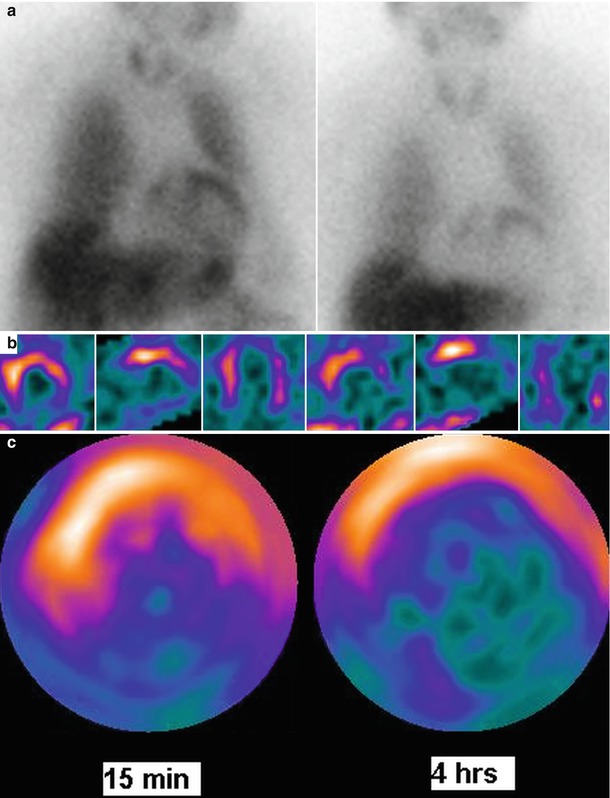
Figure 9-9.
In patients with CADN, myocardial 123I-MIBG uptake is decreased and heterogeneous [28]. Furthermore, some scintigraphic investigations have shown that in both T1DM and T2DM, cardiac sympathetic disturbances are more frequent than when assessed by CART. Myocardial sympathetic derangement is related to the severity of CADN. 123I-MIBG defects initially are predominant in the LV inferior wall [28, 29] but may be complete in advanced CADN. This figure corresponds to cardiac 123I-MIBG imaging of a patient with T2DM, at 15 min and 4 h of tracer injection: (a) anterior planar images, (b) mid-LV axial SPECT slices, and (c) SPECT polar maps, showing 123I-MIBG defects mainly in the apical and inferior LV walls.
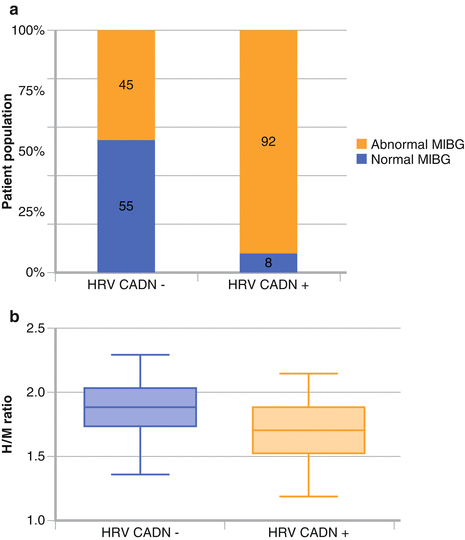
Figure 9-10.
(a, b), Various studies have shown cardiac sympathetic dysinnervation both in patients with and in those without CART indicative of CADN, suggesting that cardiac 123I-MIBG imaging is a more sensitive means to detect CADN, particularly in its early stages [30, 31]. Atherosclerosis of the large coronary arteries is not a prerequisite for these findings because 123I-MIBG defects are present even in the absence of CAD; however, the role of microvascular disease has not been well defined (Adapted from Scholte et al. [31]).
123I-MIBG Imaging in the Assessment of Prognosis
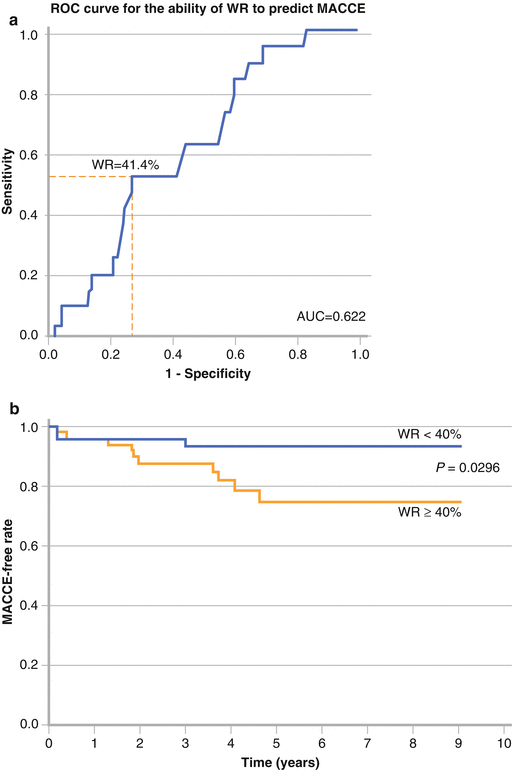
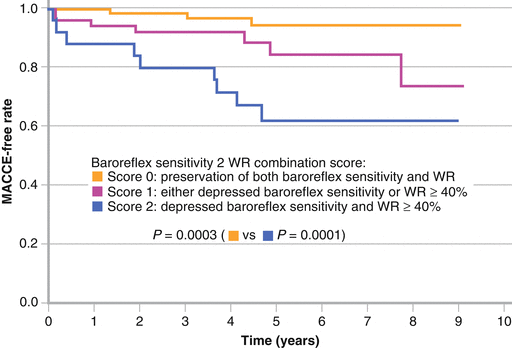
Figure 9-11.
Several studies associated decreased and heterogeneous myocardial 123I-MIBG uptake with increased mortality or other markers of adverse prognosis in patients with DM. Yufu et al. [32] reported that an abnormal WR of cardiac 123I-MIBG at baseline had long-term cardiovascular predictive value in Japanese T2DM patients without structural heart disease or severe DM complications. The incidence of major adverse cerebral and cardiovascular events (MACCE), including cardiac death, coronary revascularization, stroke, and heart failure (HF) requiring admission, was significantly higher in patients with an abnormal WR than in those with a preserved WR during a follow-up of 4.6 years. Cox proportional hazards regression analysis showed that age and abnormal WR (defined by the receiver-operating characteristic [ROC] curve in panel a) were significantly and independently associated with the incidence of MACCE (panel b; HR, 4.06; 95 % CI, 1.194 − 18.76) (Adapted from Yufu et al. [32]).