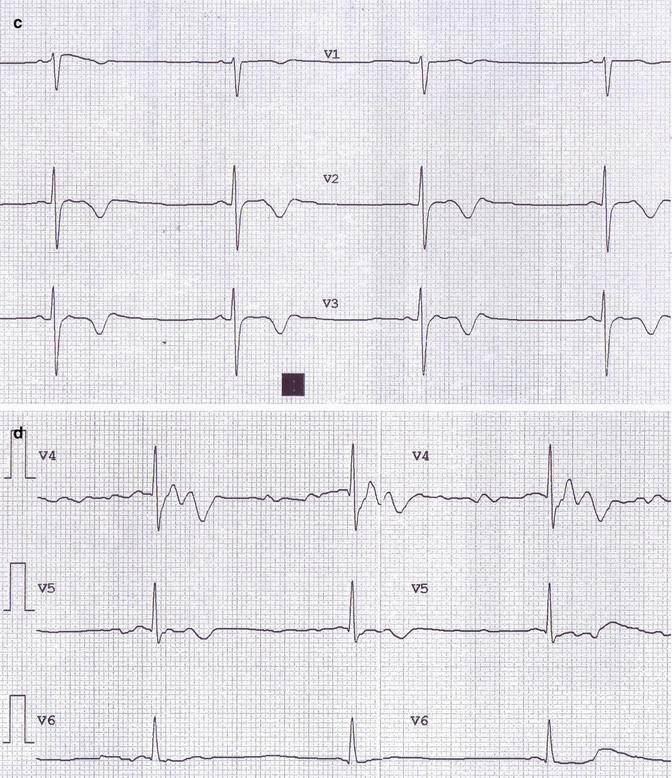
Fig. 29.1
(a–d) Image 1 ECG: sinus bradycardia, heart rate of 36 b/min, and negative T waves from V1 to V5
ECG showed marked sinus bradycardia (heart rate was 36 b/min), normal atrioventricular and intraventricular conduction, and diffuse alterations of ventricular repolarization with negative T waves from V1 to V5; QTc = 435 msec.
What Are the Possible Causes of Agitation and Dyspnea in This Patient? Why Are There Sinus Bradycardia and Diffuse Alterations of Ventricular Repolarization?
Acute coronary syndrome (ACS)
Electrolyte disorders (hypokalemia, hyponatremia)
Drug intoxication
Exacerbation of renal failure
ACS is very unlikely because the patient didn’t present chest pain, there was a little movement of cardiovascular enzymes (probably secondary to worsening of renal function), and an echocardiogram showed no alterations in the kinetics. Blood tests showed slight alterations of sodium and potassium that did not cause any electrocardiogram abnormalities.
Instead, there was a marked alteration of lithium levels (normal values, 0.6–1.2 mEq/L for chronic bipolar disorder; 1.0–1.5 mEq/L in the case of acute manic episode) that could explain both agitation state and ECG changes.
Retention of lithium is favored by all those conditions that may be associated with intravascular volume depletion.
In this case, gastroenteritis has led to a state of dehydration which resulted in the deterioration of renal function and an increase of lithium serum levels.
The patient was monitored and a fluid infusion was set up with saline to restore an adequate blood volume and hydration state.
After a few hours, there was a gradual reduction in blood creatinine and lithium levels with the regression of the electrocardiographic changes shown above (Fig. 29.2a–d).
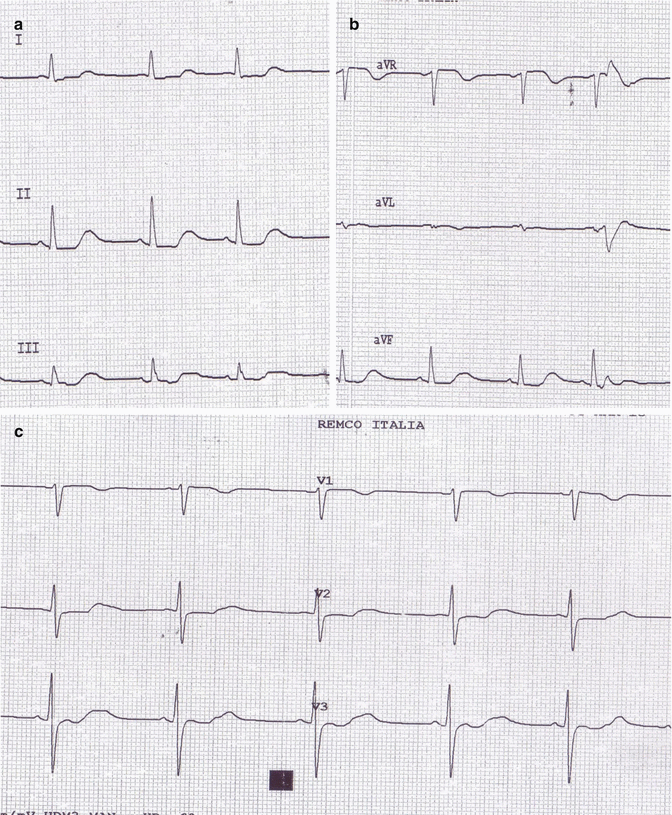
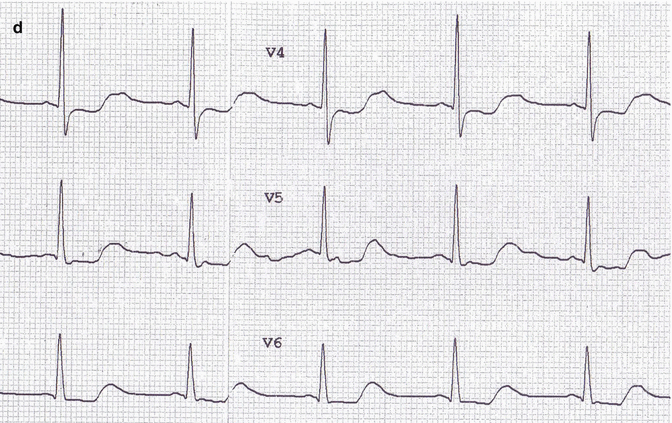


Fig. 29.2
(a–d) Image 2 ECG: reduction of previous electrocardiographic changes
The patient was discharged completely asymptomatic the next day, with a normal electrocardiogram and with good renal function. We advised a further psychiatric check for a possible optimization of the specific medical therapy.
Lithium
Lithium salts are still used in the prophylaxis and treatment of bipolar syndrome (manic–depressive) with a dose ranging from 1 to 1.5 g per day in the attack phase to 300–400 mg daily as a maintenance dose. Lithium is still considered the drug of choice in the treatment of patients with “affectivity bipolar,” that is, for people who experience alternate phases and periods of sadness and hopelessness (depression) with periods of excitement and euphoria (mania).
Lithium salts have a narrow therapeutic index, and their use should always be subordinate to the possibility of periodic blood tests.
The daily dose of lithium should be individualized on the basis of the drug plasma levels. It is important to determine the plasma concentration of lithium (lithium levels) once per week for the first 2 months, once a month for the next 6–8 months, and once every 2–3 months during the maintenance phase of therapy. Lithium levels should be checked after each change of the dose.
Clinical signs of toxicity manifest themselves at concentrations of lithium levels equal to or higher than 1.5 mEq/l. The majority of patients reach levels of toxicity when intercurrent diseases (diarrhea, vomiting, heart failure, renal failure) or drug interactions (NSAIDs, ACE-I) are present.
Side Effects
Lithium can cause a number of side effects in various districts of the organism (see Table 29.1).
Table 29.1
Lithium side effects
Side effects |
|---|
Nervous system disorders |
Absences, seizures, drowsiness, dizziness, fatigue, lethargy, psychomotor delays, confusion, restlessness, stupor, coma, tremors (twitching, clonic movements of the legs), ataxia, dry mouth |
Nephrology |
Decreased ability to concentrate urine, renal failure (rarely), diabetes insipidus |
Endocrinological |
Hypothyroidism, hyperparathyroidism, weight gain (insulin-like effect) |
Sense organs |
Taste changes, alteration of thirst |
Teratogenic |
Likely association with Ebstein’s anomaly (avoid use during pregnancy) |
It is also known that lithium can cause various cardiac disorders, including conduction abnormalities. The mechanisms underlying these conduction defects are not clearly understood. A wide range of lithium toxicities have been observed, from severe bradycardia to arrhythmias. Direct effects on the sinus node and thyroid function abnormalities to underlie these side effects have been postulated. However, most previous cases of conduction abnormalities occurred in the setting of chronic lithium therapy or in patients receiving toxic levels of lithium [1].
The major cardiovascular side effects of lithium include:
Unmasking of Brugada syndrome
Sinus node dysfunction
Atrioventricular (AV) block
Various arrhythmias (torsades de pointes, ventricular tachycardia, ventricular fibrillation)
Asymptomatic electrocardiographic changes (changes in T waves characterized by flattening, isoelectrical changes, or inversion)
Prolongation of the QT interval
Although most side effects occur at the supratherapeutic levels, there are several case reports of conduction abnormalities induced by lithium in patients at levels within the therapeutic range. This suggests a wide range of individual responsiveness to lithium [1].
29.2 Drug-Related ECG Adverse Events
Several drugs and toxins can act on the cardiovascular system, either at therapeutic level or secondary to overdose and poisoning. They may cause ECG abnormalities, heart rhythm disturbances, depression of myocardial contractility, and vasodilation.
There are two main mechanisms underlying the adverse cardiovascular effects of pharmacological and toxin agents:
A direct action on ion channels: sodium (Na+) channel blockers, outward potassium (K+) channel blockers, calcium channel blockers (CCB), sodium–potassium adenosine triphosphatase (Na+/K+ ATPase) blockers
Effects on the autonomic nervous system: vagal and sympathetic action and beta-adrenergic block
Drugs cause ECG changes also indirectly, determining electrolyte and acid–base imbalance. It must be remembered that many drugs produce effects resulting from the combination of several mechanisms.
Drugs and Toxins Acting on Ion Channels
Sodium Channel-Blocking Agents (Table 29.2)
Table 29.2
Sodium channel-blocking drugs
Cardiovascular drugs: class Ia antiarrhythmic drugs, class Ib antiarrhythmic drugs, class Ic antiarrhythmic drugs, verapamil, diltiazem, propanolol |
|---|
Psychiatric drugs: carbamazepine, cyclic antidepressants, antipsychotics (loxapine), neuroleptics (thioridazine, mesoridazine), citalopram |
Antihistamines (diphenhydramine) |
Illicit drugs: cocaine |
Other drugs: amantadine, chloroquine, hydroxychloroquine, orphenadrine, propoxyphene |
Toxins: quinine, saxitoxin, tetrodotoxin |
During phase 0 of the action potential, the opening of fast sodium channels causes Na+ influx and depolarization of cardiac cell membrane; this led to contraction of ventricular myocytes, which is expressed by QRS complex in ECG.
Inhibitors of fast Na+ channels slow the rise of the depolarization in phase 0; in case of overdose, there is a progressive widening of the QRS complex, with the risk of heart block and ventricular arrhythmias. The depression of myocardial contractility is responsible for hypotension induced by these agents [2].
Class I antiarrhythmic drugs belong to this category.
Class Ia antiarrhythmic drugs are quinidine, procainamide, and disopyramide. They are potent inhibitors of sodium channels and additionally determine a block of potassium channels.
At high plasma concentrations, conduction through the AV junction is slowed and the automaticity and refractoriness of His–Purkinje system are reduced; QT prolongation is the first sign in ECG and is related to the increased duration of the action potential in ventricular muscle fibers; with increasing doses, there is a progressive flaring of the QRS complex.
Sinus node dysfunction, varying degrees of AV block, ventricular arrhythmias, and torsades de pointes (TdP) can also occur; also during the first administrations, quinidine can cause TdP, in a dose-independent manner.
Quinidine and disopyramide at high doses have a potent negative inotropic effect, resulting in hypotension and shock. Toxic levels of quinidine are responsible for a syndrome known as cinchonism, with tinnitus, blurred vision, photophobia, confusion, delirium, and abdominal pain.
Procainamide toxicity is similar to that of quinidine but with less negative inotropic effects [3–5].
Class Ib antiarrhythmic drugs have a high affinity for sodium channels that are in the inactivated state, with a rapid binding kinetic on–off. They reduce the duration of the ventricular action potential but do not act on potassium channels and therefore do not affect the QT interval [6].
Since lidocaine easily crosses the blood–brain barrier, neurological effects are the first to appear in the case of toxicity, with light-headedness, seizures, confusion, hallucinations, and comatose state. At cardiac level, there is a slowing of conduction and reduction in myocardial contractility, and with very high doses, asystole, advanced heart block, and refractory hypotension may occur [5].
Class Ic antiarrhythmic drugs (flecainide, propafenone, encainide) bind to sodium channels in the activated state. At high plasma concentrations, these agents have a potent negative inotropic effect; the PR interval and QRS duration are prolonged, and QT interval generally is not very affected.
Propafenone unlike the other drugs in this class also possesses subclinical beta-blocker properties [5].
Cyclic antidepressants block sodium channels in a manner similar to quinidine, slowing the rise of phase 0 of the action potential; in case of toxicity, an increase in duration of QRS and QT interval can occur, with a depression of myocardial contractility [6].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


