Chapter 17 Drug-Induced and Iatrogenic Respiratory Diseases
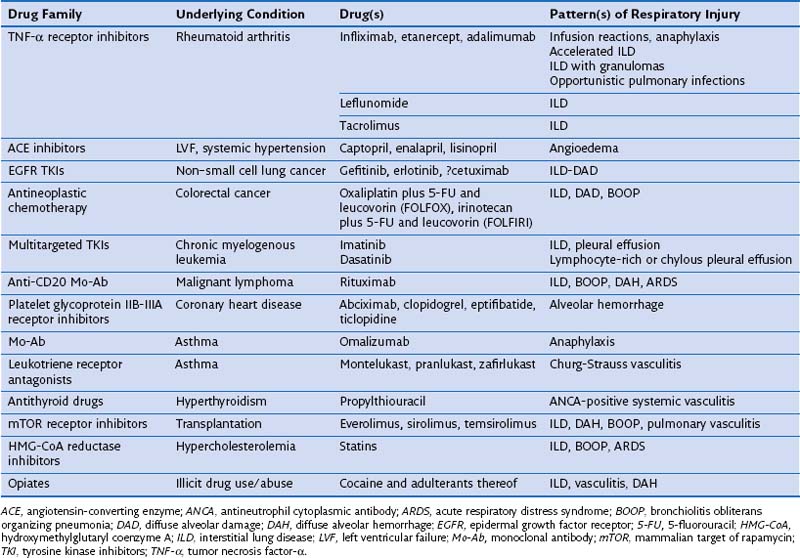
An ever-increasing number of therapeutic drugs (n = 455), hormones, and investigational compounds have been associated with severe adverse respiratory reactions in adults, children, health care professionals, and persons who work in the drug industry or otherwise handle drugs, as well as in companion animals, horses, and cattle. A periodically updated online list of causal drugs is maintained on the Pneumotox website (www.pneumotox.com), sponsored by the GEPPI (Groupe d’Etudes de la Pathologie Pulmonaire Iatrogène) investigators. Agents of recent interest or concern are listed in Table 17-1. Adverse effects of drugs such as amiodarone, antineoplastic chemotherapy agents, nitrofurantoin, or nonsteroidal antiinflammatory drugs (NSAIDs) are still commonly observed. Owing to their less trendy nature, however, the number of publications dealing with these medications represents an underestimate of the true incidence. With the recently renewed interest in nitrofurantoin for use as a urinary antiseptic agent, a resurgence of new cases of nitrofurantoin lung may potentially be expected. The same holds true for thalidomide. The clinical problem of drug overdose, although capable of producing severe pulmonary edema, is beyond the scope of drug-induced lung disease in this chapter.
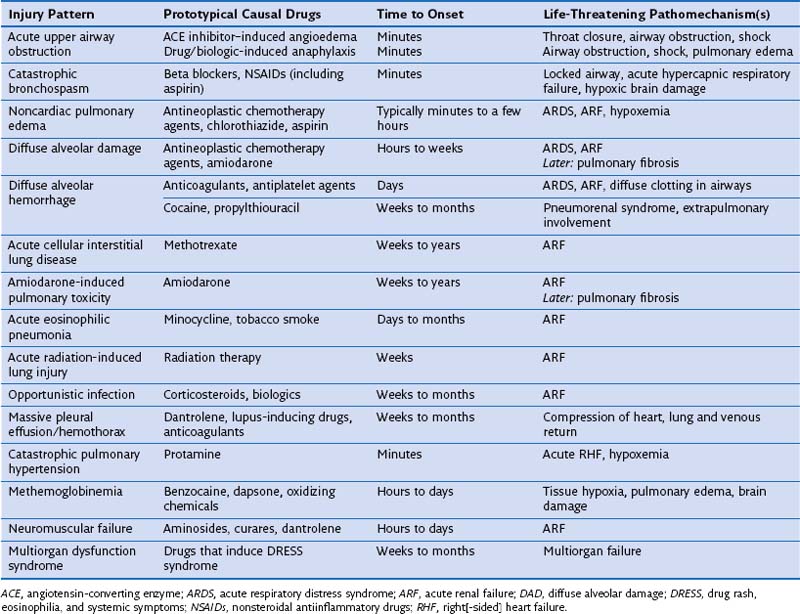
DIRD may be detected at a subclinical stage on imaging or bronchoalveolar lavage (BAL) fluid analysis, or it can be clinically evident and serious, requiring prompt recognition, immediate drug withdrawal, and emergent management aimed at securing airway patency, restoring gas exchange, and correcting hemoglobin dysfunction. DIRD presentations that portend particular severity (Table 17-2) include drug-induced anaphylaxis, angioedema, and bronchospasm, which may cause acute airway obstruction; NCPE; diffuse alveolar damage (DAD); DAH; extensive ILD, including eosinophilic pneumonia; opportunistic infections including Pneumocystis and viral pneumonias; massive pleural effusion; methemoglobinemia; neuromuscular failure; and multiorgan dysfunction. Drug-induced and iatrogenic events that occur intraoperatively, including drug-induced explosive coughing, curare- or latex-induced anaphylaxis, opiate-induced pulmonary edema, transfusion-related acute lung injury (TRALI), protamine-induced acute pulmonary hypertension, or negative-pressure pulmonary edema, raise difficult diagnostic and management issues. Awareness and emergent management are essential; however, some DIRDs are not survivable (see Table 17-2).
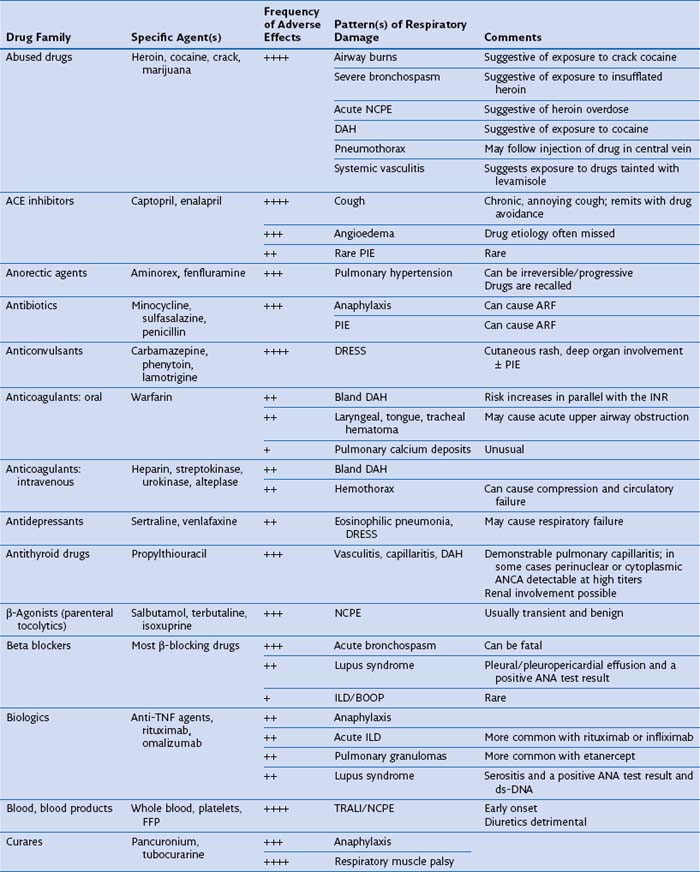
Several drugs within certain pharmacologic classes—curares, opiates, angiotensin-converting enzyme (ACE) inhibitors, β-adrenergic receptor blockers, antidepressants, appetite suppressants (anorectics), anticonvulsants, antineoplastic chemotherapy agents, ergots and ergolines, leukotriene receptor antagonists (LTRAs), mTOR (mammalian target of rapamycin) inhibitors, NSAIDs, and statins—may cause similar kinds of DIRD, suggesting a common pharmacologic cytopathic mechanism (Table 17-3). Furthermore, patients who exhibit an adverse reaction from one drug are at risk for cross-reaction if challenged with another drug of the same family. In the case of anticonvulsants, relatives of patients with the syndrome of drug rash, eosinophilia, and systemic symptoms (DRESS) may be at risk for development of a similar complication with exposure to this class of medications.
Incidence and Risk Evaluation
Pharmacokinetics and sequestration of the drug—amiodarone, desethyl-amiodarone, bleomycin, mTOR inhibitors
Metabolic activation of drug in lung cells—paraquat and possibly nitrofurantoin
Cumulated drug dosage—amiodarone, bleomycin, nitrosoureas
Sequential exposure to the drug, an important consideration when retreatment is being considered—bleomycin, nitrosoureas
Combinations of pneumotoxic drugs—gemcitabine plus bleomycin, gemcitabine or other chemotherapy agent plus radiation therapy to the chest
Cytochrome P-450 variant allele carrier status—oral anticoagulants, cocaine, and possibly other drugs
Impurities formed during drug synthesis—“peak E” contaminant of L-tryptophan
Extremes of age—amiodarone or bleomycin in older people, nitrosoureas in younger persons
History of smoking—amiodarone, bleomycin
Gender—amiodarone in male patients, bischloroethyl nitrosourea (BCNU) in female patients
Renal failure—bleomycin, amiodarone
Preexisting lung disease—amiodarone, DMARDs including methotrexate, leflunomide, anti-TNF agents, gefitinib
Atopy—minocycline, NSAIDs, drugs causing hypersensitivity or anaphylaxis
Ethnicity—enhanced risk for development of ACE inhibitor–induced angioedema in dark-skinned people, and of ILD in association with use of gefitinib, bortezomib, leflunomide, and tacrolimus in persons of Japanese ancestry
Diagnosing Drug-Induced Respiratory Disease
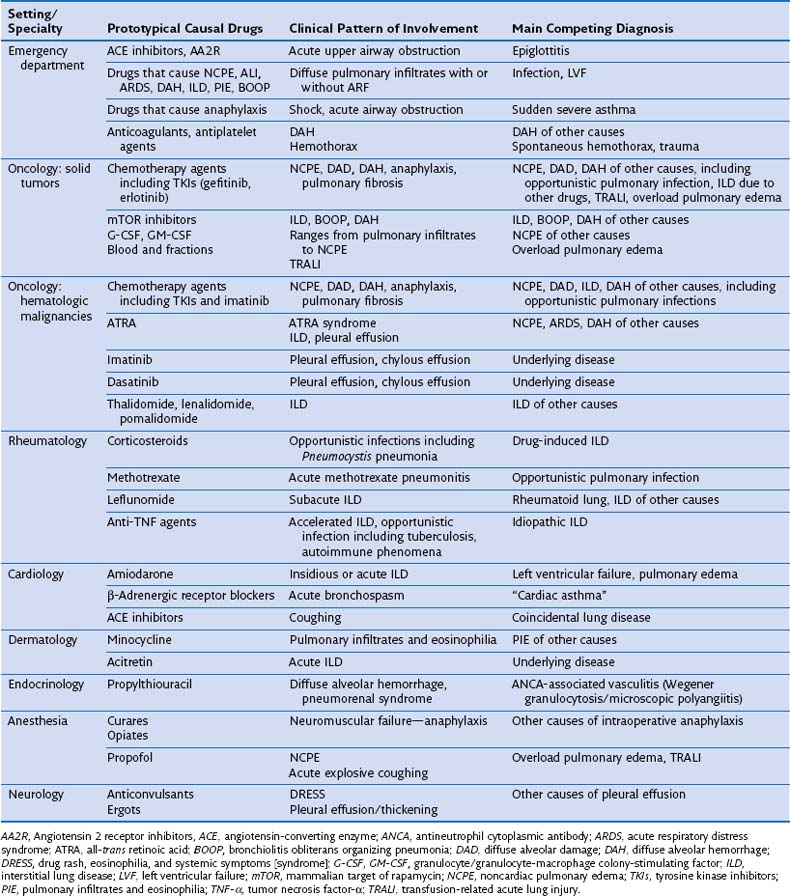
Diagnosing DIRD accurately is essential with regard to decisions to discontinue or continue the presumptively offending agent, because inappropriate drug withdrawal may have a negative impact on outcome, and because rechallenge may lead to fatal relapse. Issues regarding the diagnosis of DIRD and the corresponding differential diagnosis differ according to the underlying illness and specific clinical context (Table 17-4). Particularly difficult issues arise in patients with solid tumors, hematologic malignancies, or rheumatoid arthritis and in recipients of bone marrow or hematopoietic stem cell transplants, who are immunosuppressed under the combined influence of the underlying condition and the drugs (including corticosteroid therapy) and the radiation used to treat it.
The evidence base for respiratory disease as being drug-induced is wide-ranging, because the literature on DIRD consists mostly of case reports and is subject to reporting bias. There is a paucity of epidemiologic studies showing a convincing association of exposure to a drug and occurrence of pulmonary events. Investigating a case of possible DIRD requires a high degree of awareness, careful exclusion of conditions that DIRD can resemble (e.g., Pneumocystis or viral pneumonia with use of BAL), cessation of administration of the suspected agent (underlying disease permitting), and discussion of whether corticosteroid therapy is indicated, bearing in mind that corticosteroid therapy itself may complicate evaluation of the effect of drug withdrawal on signs and symptoms (drug withdrawal may be without an effect in patients with explosive or severe presentations). Evidence indicating that a respiratory reaction is drug-induced consists of the following: (1) the likelihood of exposure to a compatible drug, particularly if given as a solo agent, (2) previous conclusive reports of reactions to the drug, (3) pattern of involvement compatible with the specific drug, (4) compatible temporal relationship of exposure and onset and progression of symptoms, best demonstrated in patients who experience an acute reaction such as acute bronchospasm, anaphylaxis, angioedema, or pulmonary edema; (5) abatement of signs and symptoms after cessation of drug administration; (6) supportive findings on BAL fluid and histopathologic analysis; (7) lack of an alternative diagnosis after meticulous investigation using BAL and, in selected cases, lung biopsy; and (8) relapse of signs and symptoms on rechallenge, a potentially hazardous test. Box 17-1 summarizes these diagnostic criteria and presents a useful approach to diagnosis and management of DIRD.
Box 17-1
Approach to Diagnosis and Management of Drug-Induced Respiratory Disease
Diagnostic Criteria
 Confirmed exposure to an eligible drug
Confirmed exposure to an eligible drug
 Pattern of reaction appropriate for the drug
Pattern of reaction appropriate for the drug
 Appropriate latency period and timing of onset of respiratory symptoms relative to taking the drug
Appropriate latency period and timing of onset of respiratory symptoms relative to taking the drug
 Supportive BAL and histopathologic findings
Supportive BAL and histopathologic findings
 Resolution of symptoms after dechallenge
Resolution of symptoms after dechallenge
 Exclusion of any other cause including other drugs
Exclusion of any other cause including other drugs
Checklist for Fine-Tuning Diagnosis and Management
1. Depending on level of consciousness and presence or level of respiratory distress, take history of exposure to drugs, occupational/environmental agents, chemicals, and toxic substances. Confirm drug history from relatives, friends, or pharmacist when needed.
2. Evaluate risks associated with discontinuance of drug for underlying condition. Substitute when appropriate.
3. Arrange for timely evaluation of drug and metabolites in blood and/or urine (e.g., opiates, amiodarone, aspirin, mTOR inhibitors) and a urine drug screen.
4. List patterns of respiratory involvement attributable to an underlying disease if present. Match with pattern of involvement from the drug or drugs under consideration. Check for overlapping features.
5. Check complete blood count, CD4+ count, autoimmunity (ANA, ANCA), and HIV serostatus whenever appropriate.
6. Retrieve earlier, pretreatment, and baseline imaging studies, respiratory physiology reports, laboratory data, and autoantibody assay results.
7. List exposure to any drug (even if remote), blood transfusion, blood products, radiation therapy, concealed exposure to illicit drugs, substances of abuse, and chemicals used to synthesize drugs in the household.
8. List possible risk factors related to dosing or blood levels (see text).
9. Examine timing of exposure—discontinuation versus latency period, onset, peak, and resolution of pulmonary symptoms and signs on imaging studies.
10. Match clinical presentation, imaging data, and findings on BAL, laboratory testing, ANA and ANCA assays, and histopathologic analysis (if available) with the drug under study. Contribution of KL-6 glycoprotein assay and in vitro tests is limited.
11. Examine other diagnostic possibilities including Pneumocystis and viral pneumonia and pulmonary involvement from the underlying disease.
12. Check laboratory findings for involvement of organs other than the lung.
13. If patient has been exposed to more than one drug, assess causality for each specific drug.
14. Examine whether adverse reaction may result from the known pharmacologic effect of the drug
15. Determine pharmacogenetic trait when appropriate.
16. Evaluate whether corticosteroid therapy is indicated in addition to drug withdrawal (as indicated by clinical severity).
17. Schedule follow-up assessment for signs/symptoms and imaging and laboratory abnormalities after drug withdrawal, to confirm positive dechallenge.
18. Ensure proper communication with any health professional so that the patient is not inadvertently rechallenged with the drug.
19. Discuss whether deliberate rechallenge is indicated (reserved for vital drugs for which no substitute is available). This procedure is performed using low doses in a hospital setting close to an ICU. Tolerance can be induced for a limited number of drugs.
20. Report to national/federal drug safety monitoring authorities; plan publication of findings.
Patterns of Drug-Induced Respiratory Disease
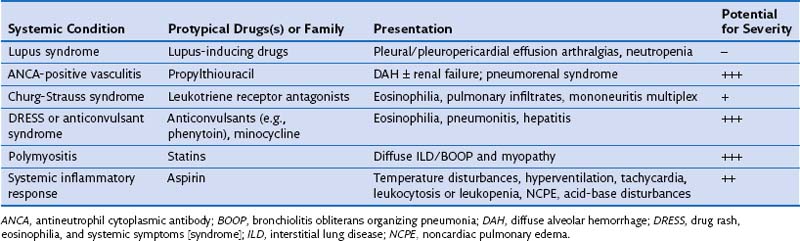
DIRD may at times manifest as a systemic condition with extrapulmonary and pulmonary involvement. Systemic presentations include the drug-induced lupus syndrome, antineutrophil cytoplasmic antibody (ANCA)-positive vasculitis with DAH, and the Churg-Strauss syndrome. In these conditions, involvement of the skin, kidney, heart, and lung occurs in a manner similar to that seen in the idiopathic conditions. The DRESS syndrome includes a distinctive cutaneous rash and involvement of the liver, kidney, central nervous system, fever, and blood eosinophilia; in a fraction of affected patients, pneumonitis is present. Propythiouracil and anticonvulsants epitomize the culprit agents for drug-induced ANCA–related vasculitis and DRESS, respectively (Table 17-5).
Parenchymal Patterns
Nonspecific Interstitial Pneumonia: Cellular Interstitial Pneumonitis
On histopathologic examination, the pattern of NSIP consists of interstitial inflammation with expansion of alveolar septa by a mild to moderate mononuclear cell infiltrate, occasional eosinophils, and interstitial edema. Among the well over 100 drugs that may cause this condition, β-blocking drugs, amiodarone, flecainide, fludarabine, gold salts, imatinib, lenalidomide, methotrexate, mTOR inhibitors, nilutamide, nitrofurantoin, propylthiouracil, statins, sulfasalazine, thalidomide and its congeners, tocainide, and venlafaxine are cited most often (see under categories Ia and Ib on the Pneumotox website). Methotrexate pneumonitis in rheumatoid arthritis seems to typify acute drug-induced NSIP. Collection of evidence for drug relatedness can be done using the approach set forth in Box 17-1.
Onset of signs and symptoms can be insidious or rapid, with a dry cough, dyspnea, fever, fatigue, and malaise (Figure 17-1). In patients who develop this complication with methotrexate and particularly if the drug is continued, the disease may accelerate without notice, causing further shadowing and progressive respiratory failure. The chest radiograph exhibits areas of ground glass attenuation, confluence, or consolidation, which often predominate in the bases, where such changes may extend rather than migrate. In more advanced cases, findings include areas of consolidation with air bronchograms and generalized volume loss, which can be marked. In early or mild cases, HRCT discloses a discrete and diffuse haze, ground glass shadowing, or mosaic attenuation, which may resemble hypersensitivity pneumonitis in appearance. Other signs on imaging include disseminated or diffuse inter- and/or intralobular septal thickening or “crazy paving”; in severe cases, an associated pleural exudate can be present. The BAL fluid typically reveals a CD8+-dominant lymphocytosis, except with bacillus Calmette-Guérin (BCG)-induced pneumonitis, and sometimes an associated increase in neutrophils and/or a modest increase in eosinophil counts. The results of BAL fluid analysis are influenced by timing of the test in the course of the disease and whether the patient has received corticosteroids. BAL has an important exclusionary role: It is used to rule out a coincidental or drug-induced infection, the main competing diagnosis in drug-induced ILD. Particularly important is the use of the most recent nucleic acid–targeted molecular techniques to diagnose Pneumocystis jiroveci
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree