Fig. 24.1
Drug-eluting stent structure composed of a metallic stent, a polymer-based drug delivery platform, and a pharmacologic agent. Various available platforms, polymers, and drug agents are described
24.2 DES Structure
DESs are composed of a metallic stent, a polymer-based drug delivery platform, and a pharmacologic agent (typically an immunosuppressant and/or antiproliferative compound) (Fig. 24.1). The goal of DES technology is to minimize PCI-related vascular inflammation and cellular proliferation. Available platforms are made of stainless steel, cobalt-chrome, or platinum-chrome. Cobalt-chrome alloys provide increased radial strength and radiopacity, as compared with stainless steel, allowing for development of thinner struts with improved deliverability of the device. Another characteristic is that platforms made with thinner struts may result in less arterial injury and reduce the risk of in-stent restenosis (ISR), in turn associated with lower thrombotic risk. Platinum-chrome alloys can further improve radial strength. Polymer coatings that are applied to the stent surface serve as drug carriers and allow a controlled drug release. We discuss the progress in polymer technology aimed at decreasing local inflammatory reactions and thrombosis by improving the biocompatibility of polymers. DESs that have been approved by the Food and Drug Administration (FDA) have durable polymer coatings. However, new platforms for DES feature polymers that biodegrade after drug elution, resulting in a stent surface similar to that of a bare-metal stent. First-generation stents released sirolimus or paclitaxel and had stainless steel platforms, whereas new-generation stents release everolimus or zotarolimus and feature cobalt-chrome or platinum-chrome platforms with thinner strut thickness and more biocompatible, durable polymer coatings.
24.3 Challenges with First DESs
24.3.1 Neointimal Formation and In-stent Restenosis
The lack of endothelial coverage and the consequent inflammatory response in the vessel wall are able to stimulate a remodeling process with internal migration and proliferation of medial smooth muscle cells (SMCs) and deposit excess extracellular matrix (ECM) proteins that ultimately obstruct the vessel lumen [3–6]. This cascade of molecular processes can ultimately lead to development of the angiographic ISR, an intra-stent narrowing owing to new formed plaque that can require further intervention (Fig. 24.2).
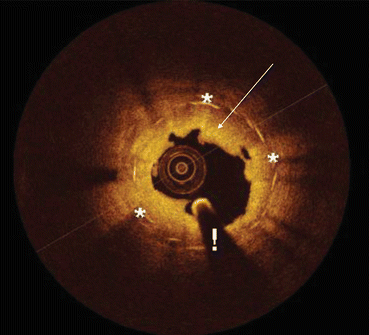

Fig. 24.2
Optical coherence tomography (OCT) image of in-stent restenosis. Restenosis is demonstrated as neointimal proliferation (arrow) beyond the stent metallic struts (*) inside the vessel lumen. OCT catheter (!)
24.3.2 Stent Thrombosis
Stent thrombosis (ST) is a catastrophic event that is associated with increased myocardial infarction (MI) rates culminating in increased mortality (Fig. 24.3). Intense controversy regarding the safety of DES has been sparkled in the last few years as long-follow-up data reports were gradually becoming available and showed an increased risk of late ST and MI in patients treated with a first-generation DES after discontinuation of dual antiplatelet therapy (DAPT). The incidence of ST up to 1 year follow-up seems similar for DES and old bare-metal stents (BMS) and ranges from 0.6 to 3.2 % for BMS and 0.6 to 3.4 % for DES, depending on patient and lesion characteristics [7–10]. Before the introduction of DES, ST was perceived as a complication occurring early after stent implantation. The typical clinical presentation of ST consists of chest pain and ischemic electrocardiographic changes in the target vessel territory, presenting in some cases as acute MI. However, ST can also manifest itself as sudden death, or it can be asymptomatic in the setting of collateral vessels. A multitude of mechanisms are potentially involved into the occurrence of ST as well as various patient-related, lesion-related, procedural, and post-procedural factors [11–19]. The suggested pathophysiological mechanisms after DES implantation that predispose to ST are the following: (1) the exposure of blood before re-endothelialization to prothrombotic subendothelial constituents, stent struts, and/or polymer material can lead to activation of the extrinsic pathway of the coagulation cascade; (2) a persistent slow coronary blood flow and low shear stress can activate the intrinsic pathway; (3) inadequate pharmacological suppression of platelet activation (e.g., after premature discontinuation of DAPT); and (4) the presence of a systemic prothrombotic state (e.g., due to acute coronary syndrome or malignancy). A meta-analysis found that most consistently reported predictors are early DAPT discontinuation, the extent of coronary artery disease, and total stent length [18].
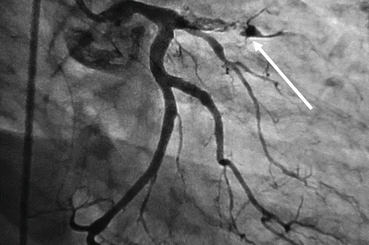

Fig. 24.3
Angiographic image of stent thrombosis. Arrow points to the distal stent portion in the proximal left anterior descending coronary artery
24.4 DES Characteristics
24.4.1 First-Generation DES
Over the past 10 years, first-generation DESs, especially sirolimus-eluting stent (SES) and paclitaxel-eluting stents (PES), have become the most widely used devices worldwide for management of coronary artery disease. However, despite their clear superiority in preventing ISR and that the need for repeat revascularization due to eluted antiproliferative drugs is certainly proven, concerns have emerged in regard to their long-term safety, strictly late and very late thrombotic events. In a network meta-analysis [20] (an analysis of studies of multiple interventions that makes use of direct and indirect comparison) involving 38 trials and more than 18,000 patients, there was a marked reduction in the rate of repeat revascularization with both SES and PES, as compared with BMS. Based on this analysis, seven patients (95 % confidence interval [CI], 6–8) would need to be treated with SES and eight patients (95 % CI, 7–10) with PES in order to prevent one repeat revascularization, as compared with BMS. However, stents that release sirolimus or paclitaxel have been associated with an increased risk of very late ST, as compared with BMS [10, 21]. In contrast, the risks of death and MI with SES and PES were similar to the risks with BMS [20] which may be explained by the low incidence of very late ST (annual rate, 0.2–0.6 %) and the compensatory effects of a reduced risk of ISR, which is manifested as MI in 10–20 % of patients [22, 23]. The lack of a significant difference in mortality or MI between first-generation DES and BMS despite the increased risk of very late ST with DES may be because ISR is not always a benign phenomenon, presenting as acute MI in 3.5–19.4 % of patients. Thus, a small increase in a low-frequency event (late or very late ST) with frequent, serious, life-threatening consequences may be blunted by a large reduction of a more common event (ISR), which is more rarely associated with serious clinical consequences.
24.4.2 DESs Versus BMSs
BMSs were the first devices used for coronary stenting. Interestingly, although these devices reduced rates of restenosis compared with balloon angioplasty, ISR, classified as a narrowing within the stented segment of at least 50 %, continued to develop in up to 20–30 % of lesions [24, 25]. Although stent insertion prevents arterial recoil and stabilizes vascular dissections, ISR might still occur because of exuberant neointimal accumulation much akin to “scar formation” the mechanisms of which are discussed in detail later. In addition to acting as a vascular scaffold, stents soon evolved to become drug delivery systems in the form of modern DESs. One collaborative network meta-analysis indicated that DESs and BMSs are associated with similar rates of overall and cardiac mortality and that use of SES is associated with a reduction in the risk of MI compared with the use of BMS and PES [20]. Although there was little evidence of an overall increase in definite ST associated with DESs, the authors found PES to be associated with an increased incidence of late ST compared with BMS and SES. Wide credibility intervals precluded definite conclusions on a potential increase of late ST with SES compared with BMS. A secondary analysis showed a marked reduction in target lesion revascularization (TLR) with both DES, which was more pronounced for SES than for PES. Lastly, little evidence was found of an increased risk of mortality associated with either DES in diabetic patients, but wide confidence intervals again precluded definite conclusions.
A recent series of pooled analyses of randomized trials comparing DES with BMS found preliminary evidence for an increased risk of late ST (later than 30 day occurrence) associated with DESs as compared to BMS [8, 10, 26, 27]. These analyses included between 4 [10, 26] and 14 [27] trials and between 1,748 [10, 26] and 4,958 [27] patients. A pooled analysis of four trials including 428 diabetic patients by Spaulding and colleagues [26] found a significant increase in mortality with SES compared with BMS. These results are difficult to interpret: the number of patients was small and the mortality rate of diabetic patients was surprisingly low among those with BMS. Despite the statistical significance, chance could have contributed to Spaulding and colleagues’ results. The increase in the risk of Academic Research Consortium (ARC)-defined definite late ST that was found for PES compared with BMS was lower—but more precise—than the increase reported in a pooled analysis by Stone and colleagues [10]. The use of per-protocol definitions for ST could have resulted in an overestimation of the risk increase associated with PES. Late ST occurs less frequently with SES than with PES, which is concordant with a recent observational study by Daemen and colleagues [28]. With regard to target lesion revascularization, the results are mainly driven and compatible with those from the two largest trials—REALITY [29] and SORT OUT II [30]—which failed to show a significant difference in TLR between the two drug-eluting stents. Confidence intervals were large for both. SORT OUT II did not include scheduled, protocol-driven clinical follow-ups. Instead, data were ascertained from death and hospital registries, which could have resulted in diagnostic misclassification and biased estimates of differences between the two DESs [31].
24.4.3 Second-Generation DES
Second-generation DESs offer numerous improvements over their first-generation counterparts. Namely, second-generation devices have decreased strut thickness, improve flexibility/deliverability, enhanced polymer biocompatibility/drug elution profiles, and superior re-endothelialization kinetics. In contemporary practice, second-generation devices are now the predominant coronary stents implanted worldwide [32].
24.4.3.1 Endeavor® Zotarolimus-Eluting Stent
The Endeavor zotarolimus-eluting stent (ZES; Endeavor) is a second-generation stent based on a stronger cobalt-chromium stent platform, with improved flexibility and decreased stent strut size. In addition, the ZES uses a novel phosphorylcholine polymer coating with stable, lipid membrane analogue designed to maximize biocompatibility and minimize inflammation associated with previous polymers. As well, the polymer is engineered to shorten the drug elution time such that most of the drug is eluted during the initial injury phase. Zotarolimus is a sirolimus analogue with similar immunosuppressant properties but enhanced lipophilic properties. This key difference was featured to enhance vessel wall localization and minimize the dispersion into the circulation [33]. Indeed, preliminary animal models supported the potential benefits of this novel stenting system, resulting in less local inflammation and improved re-endothelialization compared with SESs and PESs [34]. The ENDEAVOR I trial was the first to demonstrate safety and efficacy of ZESs in humans [35]. The ENDEAVOR II trial compared the ZES with the Driver BMS, showing improved major adverse composite events (MACE) at 2 years [36]. The subsequent ENDEAVOR III trial then compared ZES with SES, with the ZES paradoxically showing greater in-stent late lumen loss (ISLL) and IRS (11.7 % vs. 4.3 %) but less MACE (0.6 % vs. 3.5 %) [37]. Long-term follow-up to 5 years displayed a “catch-up” phenomenon whereby rates of in-stent restenosis increased in SES patients to levels comparable with ZESs [38]. Similar in design, the ENDEAVOR IV trial compared ZES with PES and again found higher rates of ISR in the ZES group [39]. These findings persisted for 3 years, but clinical outcomes, mainly because of fewer MIs, were less common with the ZES, thereby suggesting a potential benefit in regard to vascular healing [40]. However, these trials were underpowered to adequately assess differences in ST. The PROTECT trial specifically addressed the incidence of ST in a randomized study of ZESs versus SESs in more than 8,700 patients followed up to 3 years and failed to demonstrate a difference in definite or probable ST rates between Endeavor and Cypher stents [41].
24.4.3.2 Resolute® Zotarolimus-Eluting Stent
The Resolute represents a refinement of the Endeavor stent, using the same cobalt-chromium (Driver) stent platform and zotarolimus agent but with a novel trilayered polymer. Similarly, the newer Resolute Integrity (sometimes classified as a third-generation DES) uses the same drug and novel trilayered polymer but is based on the new Integrity stent platform providing improved deliverability. This novel trilayered polymer is composed of three main components: a hydrophilic polymer for biocompatibility, a hydrophobic polymer for drug elution control, and a polyvinyl polymer which rapidly releases the drug immediately after implantation. The net effect is the suppression of the initial inflammatory response, followed by most of the drug being eluted over the next 60 days in an attempt to improve the late healing characteristics. The RESOLUTE trial was the first clinical study to evaluate the Endeavor Resolute and enrolled patients with simple de novo lesions in a prospective, single-arm, nonrandomized trial demonstrating clinical outcomes similar to its predecessors with no cases of ST [42]. The RESOLUTE All-Comers trial then compared the Resolute with the Xience V (everolimus-eluting stent [EES]). This study population contained greater lesion complexity and demonstrated non-inferiority of the Resolute system in terms of target lesion failure (cardiac death, target vessel MI, ischemia-driven target lesion revascularization) [9]. In the recent DUTCH PEERS randomized trial, the zotarolimus-eluting stent was non-inferior to the everolimus-eluting stent with respect to the primary endpoints of target vessel failure defined as a composite of safety (cardiac death or target vessel-related MI) and efficacy (target vessel revascularization) at 12 months (absolute risk difference 0.88 %, 95 % CI −1.24–3.01 %; upper limit of one-sided 95 %, CI 2.69 %; non-inferiority p = 0.006). Both stents were similarly efficacious and safe and provided excellent clinical outcomes [43].
24.4.3.3 Everolimus-Eluting Stent
Everolimus, a derivative of sirolimus, is a cell cycle inhibitor designed to overcome the physicochemical properties that rendered the oral administration of sirolimus difficult [44]. Similar to its predecessor, everolimus inhibits SMC proliferation in vitro and vascular intimal thickening in animal transplant models [45]. Its cytostatic properties rendered it a potentially valuable addition to the evolving arsenal against ISR, prompting the development of the Xience V/Promus CoCr-EES in parallel to the ZES as another second-generation DES. In 2004, Grube et al. published the prospective, randomized, single center, the FUTURE I feasibility trial, demonstrating safety and improved in-stent late loss (ISLL) (i.e., narrowing of the stented segment) over BMSs at 12 months [46]. This was followed by the SPIRIT FIRST trial demonstrating similar results with EES versus BMS in de novo coronary lesions [47]. Later, the SPIRIT II trial demonstrated improvements in ISLL and neointimal volumes over the Taxus PES [48]. Similarly, the SPIRIT III trial compared the Xience V and Taxus Express demonstrating improvements in late lumen loss and lower MACE rates largely because of a few MIs [49]. The subsequent second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE) trial demonstrated improved stent and clinical outcomes in a “real-world” experience, providing further support for the superiority of second-generation EES over their PES counterparts [7]. Finally, the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) trial demonstrated non-inferiority of EES to SES in inhibiting late loss at 9 months and clinical events at 12 months [50]. The newer Promus Element has the identical drug/polymer profile of the Xience V/Promus but offers improved deliverability with a novel platinum-chromium scaffold, demonstrating non-inferiority to the Xience V/Promus in de novo lesions [51].
In summary, regarding DESs, first-generation SESs and PESs provided major advances in the treatment of obstructive CAD with marked reductions in ISR. Second-generation stents appear to be safe and efficacious and provide a modest improvement in outcomes compared with their first-generation counterparts. This difference in outcomes was recently emphasized in a large (n = 94,384 patients) observational study. Compared with the first-generation DESs and BMSs, second-generation devices are associated with a lower risk of ISR, ST, and mortality [52] Thus, these new stent platforms represent the state of the art in DES design and form the cornerstone of modern PCI.
24.4.3.4 First-Generation Versus Second-Generation DES
A large-scale meta-analysis by Navarese et al. so far comparing first-generation versus second-generation DES [53] with 31,379 patients included showed the following findings:
1.
Second-generation EES and ZES significantly reduced the incidence of MI compared with first-generation PES.
2.
Only second-generation EES significantly reduced the odds of definite and definite/probable ST compared with first-generation DES (Fig. 24.4).
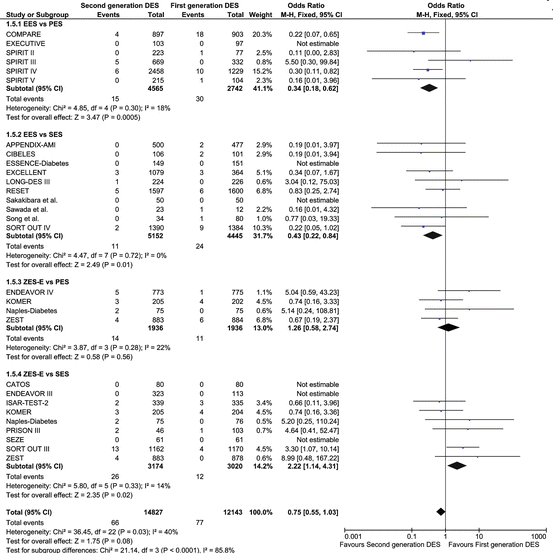

Fig. 24.4
Individual and summary odds of short-term (<1 year) definite stent thrombosis in studies comparing first- and second-generation drug-eluting stents. ORs and 95 % CIs are reported as summary statistics. The size of a square is proportional to the weight of each study (Adapted from [53])
3.
Second-generation EES and ZES-R and the first-generation SES are similar to each other with regard to their efficacy and significantly better than ZES-E and PES with regard to repeat coronary revascularizations.
Single and composite safety endpoints did not differ in direction or magnitude of the effect favoring durable polymer EES. The inflammation induced by the durable polymers of first-generation DES may result in delayed healing and incomplete covering of stent struts by new and functional endothelium with uncovered stent struts serving as a source for future episodes of ST. Secondly, other factors such as stent malapposition, mechanical tissue injury caused by stent struts during implantation, and, finally, polymer hypersensitivity or even toxicity, as is the case for PES [54] and in turn associated with persistent fibrin deposition, might also play a potential role. Second-generation DES was introduced to address the concerns raised by first-generation DES by both optimizing their metallic stent platform or polymer and eluted drug. That is, second-generation EES uses thin struts coated with durable, fluorinated polymer, which has been shown to have thromboresistant properties in experimental studies. Similarly, ZES-R combined more rapid elution kinetics than SES in the same time offering thinner, more biocompatible phosphorylcholine polymer placed on a cobalt alloy stent platform. In the meta-analysis, which compared first-generation versus second-generation DES data for most robust evidence of safety endpoints, second-generation EES was found superior to first-generation PES but not to SES. This might have been attributed to the proven overdose and/or accumulation of paclitaxel in the arterial wall due to a coronary uptake, in turn leading to toxicity, inflammation, and late in-stent stenosis, which is not the case with SES [55]. The superiority of thin strut EES and ZES in reducing the incidence of MI in the short clinical follow-up might also come from mechanistic reasons. Indeed, the positive clinical effect might be related to the more frequent side-branch jailing with thick strut devices (SES Cypher 140 μm and PES Taxus Express 134 μm vs. ZES Endeavor/Resolute 91 μm and Abbott Xience V 81 μm), resulting in turn in higher rates of periprocedural MI [56–58]. Although ST should be considered a surrogate safety endpoint, which must be interpreted in the perspectives of MI and mortality, it remains a devastating complication and is often associated with high rates of mortality and morbidity. Second-generation EES was associated with significantly lower rates of definite and definite or probable ST in short-term analysis compared with first-generation DES; this finding is in line with other meta-analyses [53, 59] showing the superiority of EES over BMS and first-generation and second-generation DES in reducing early (0–30 days) and late (31 days–12 months) ST. This analysis integrated the most updated data and enriches the previous findings of longer follow-up clinical data for particular devices, demonstrating for the first time that EES reduces definite and definite or probable ST also beyond these time frames (very late ST) compared with first-generation DES. Notably, data on EES do not reflect the performance of second-generation ZES-E in terms of stent thrombosis; indeed, Endeavor was found to even increase the incidence of definite ST as compared with SES at ≤1 year, mainly driven by the results of the SORT OUT III [60–62] and ZEST [63] trials. As zotarolimus is a synthetic analogue of sirolimus, the disparities between stents are attributed to different kinetics of drug release from the polymers used for drug elution (1 week with ZES and 3 months with SES). It is postulated that quick zotarolimus release and high initial concentrations not only affect the healing of the plaque and arterial wall but may also allow for exposure of the atheromatous debris to the bloodstream. Thus, increasing the risk of early ST, which is of particular importance in high-risk patients with acute coronary syndrome or multivessel disease. Design-related factors such as strut thickness, type of antiproliferative agent, drug elution kinetics, elution time, and type of polymer are all factors that may as well impact efficacy outcomes [64]. Although not a new finding, all limus-eluting DESs, with the exception of ZES-E, were associated with significantly lower rates of target lesion revascularization (TLR)/target vessel revascularization (TVR) than the first-generation PES. Taken together, inflammation-causing properties of paclitaxel along with the short-release curve of ZES-E preclude optimal suppression of procedure-related injury responses, in turn resulting in subsequent intimal hyperplasia and increased need for repeat revascularization [34]. Unlike ZES-E, the more recently introduced ZES-R, which has a much longer (up to 180 days) release curve than of the same antiproliferative agent, zotarolimus, is associated with a significant reduction in TVR/TLR compared with ZES-E [65]. Not surprisingly, MACE analysis confirmed the single-outcome findings with second-generation EES outperforming first-generation PES at ≤1 year and beyond. Remarkably, the initial short-term benefit of SES over ZES-E, attributable mainly to higher rates of repeat revascularization with the latter, becomes less pronounced at long-term follow-up when drug elution is over.
24.4.4 Biodegradable Polymer DES
To improve the safety of first-generation DES, new devices have been developed that use either type of biodegradable polymers combined with stainless steel platforms. Both of them have been tested in randomized controlled trials. A large meta-analysis, with 63,242 patients, examined the safety and efficacy profile of second-generation durable polymer DESs and biodegradable polymer DES (BP-DES) compared with first-generation DES and with each other [32]. Second-generation durable polymer EES and ZES-R, the first-generation SES, and the BP-DES were similar to each other concerning their efficacy and significantly better than ZES-E and PES with respect to coronary revascularizations. There was a safety gradient, with EES and ZES-R resulting in lowest rates of death and MI. Conversely, BP-DES, ZES-E, and PES were being associated with significantly increased odds of MI or stent thrombosis compared with EES. One of the most important findings was the significant increase in the odds of MI with BP-DES compared with durable polymer EES (Fig. 24.5). To date, BP-DES has been perceived as safer than first-generation SES and non-inferior to second-generation EES, mainly on the basis of results from individual trials powered only for composite endpoints of safety and efficacy [66–70]. When single (instead of composite) endpoints of safety were analyzed, the analysis provided new insights suggesting that BP-DES is associated with a similar (not higher) safety to the first-generation SES and a significantly higher rate of MI than EES. Indeed, the second-generation durable polymer EES and ZES-R were associated with the most favorable safety profile compared with not only the first-generation durable polymer PES but also the second-generation ZES-E and BP-DES.
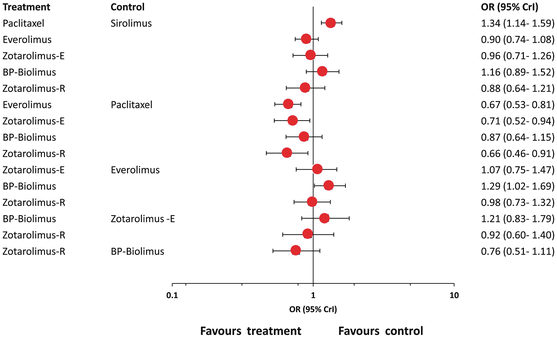

Fig. 24.5
Network meta-analysis findings on comparative efficacy of first- and second-generation drug-eluting stents (DES) as compared to biodegradable polymer DES and to each other for the outcome of myocardial infarction (MI). Analysis demonstrated significant reduction of the odds of MI with first-generation sirolimus (SES) and second-generation everolimus (EES) and Endeavor and Resolute zotarolimus (ZES-E and ZES-R) as compared to first-generation paclitaxel (PES). Additionally EES was found superior to BP-DES concerning reducing the odds of MI (Adapted from [32])
In a broader perspective, the study by Navarese et al. [32] showed that among all devices compared, the durable polymer second-generation EES and ZES-R were the safest DES to date. These findings agree with those of two previous network meta-analyses [59, 71] that compared first- and second-generation DES with BMS. However, the study substantially differed from the others by incorporating the most recent evidence from head-to-head DES comparison trials and forming the largest DES database ever analyzed, with a total of 63,242 patients. Addition of BP-DESs, which are used mainly in Europe and Asia, provided a comprehensive overview of the most widely used DES in current clinical practice worldwide, not compared so far within their class in such a scale for safety and efficacy endpoints. To date, the direct comparison between BP-DES and BMS is limited to single trials, making indirect comparisons through this “weak” common link imprecise and meaningful conclusion difficult. The safety of first-generation DES has been extensively debated. The relatively high rates of stent thrombosis associated with these devices, a phenomenon that translates into increased rates of death or MI, raised concerns regarding their widespread use, despite their clear efficacy benefits over BMS [28, 72]. Further studies showed that the mechanisms of stent thrombosis after DES implantation are complex, with factors related to device design being of paramount importance. Indeed, the inflammation induced by the durable polymers of first-generation DES could cause delayed healing and incomplete covering of stent struts by new and functional endothelium, with uncovered stent struts representing a source for future episodes of ST [73]. Other factors such as stent malapposition and mechanical tissue injury caused by stent struts during implantation, however, also play a role in stent thrombosis [7].
New-generation DESs have dealt with the limitations observed with first-generation devices in different ways; BP-DESs use abluminal biodegradable polymers that dissolve within 6–9 months, with the residual metal platform presumably regaining a safety profile similar to a BMS beyond this time frame [74]. Conversely, second-generation durable polymer DESs have replaced first-generation polymers with more biocompatible and thinner polymers [75–77]. Interestingly, the design improvements of the new-generation durable polymer DES have run in parallel with a reduction of definite stent thrombosis rates, compared with the first-generation PES and SES in early, late, and very late phases of follow-up [78]. Furthermore, late stent thrombosis with EES being the first and most studied prototype is reduced not only when compared with first-generation DES but also with BMS. This suggests that the durable fluoropolymer used in these devices might be “thromboresistant” and more biocompatible than BMS [59, 71, 79]. This in turn generates a shift from the contention of an increased risk of stent thrombosis with DES compared with BMS toward the converse relation. In contrast, BP-DES has failed to provide a significant reduction in 1 year ST rates compared with SES, with both available trials showing a numerical advantage of SES [66, 68]. Although the 5-year follow-up of LEADERS [80]—the only available trial with a long follow-up—shows a significant reduction of the 1–5-year rates of ST compared with SES, the overall rate at 5 years was not significantly lower than is for SES, pointing once more to the impact of first-year outcomes. Stent thrombosis, however, remains a surrogate safety endpoint and needs to be interpreted in the context of objective safety endpoints such as death and MI. Durable polymer DES yielded lower odds of death and MI compared with BP-DES, with EES reaching a significant reduction in MI. Of note, this finding is in line with the results of the NEXT and COMPARE II trials [69, 70] both of which showed a numerical reduction of MI associated with EES compared with BP-DES, which became significant for Q-wave MI in the latter. The advantage with regard to MI observed with thin strut devices such as EES might be related not only to ST but also to lower rates of periprocedural MI resulting from side-branch jailing, which in turn for mechanistic reasons might be more frequent with thick strut devices [56]. Higher degrees of re-endothelialization achievable with these stents compared with the thick strut devices have been shown in preclinical [57] and optical coherence tomography studies [58], which also might play a role.
These findings on safety among different DES should also be viewed in the context of patients treated with DES who need to undergo noncardiac surgery. Surgery represents one of the most common reasons for premature discontinuation of antiplatelet therapy, which is associated with a significant increase in mortality and major adverse cardiac events [81]. Indeed, the favorable profile observed with second-generation DES might become clinically relevant in this context, in light of recent studies suggesting the safety of shorter overall duration of dual antiplatelet therapy (DAPT) in patients treated with these devices [82, 83]. In this perspective, newer thin strut biodegradable polymer DES recently introduced in the market might have the potential to enhance safety and efficacy outcomes after percutaneous coronary intervention (BIO-RESORT, TWENTE III, and EVOLVE II QCA). Analyses beyond 1 year confirmed maintenance of the direction of the estimates observed at 1 year follow-up. Efficacy factors related to design, such as strut thickness, type of antiproliferative agent, drug elution kinetics, and elution time, as well as type of polymer, could all affect efficacy outcomes [64, 84].
New-generation EES, BP-DES, ZES-R, and first-generation SES were associated with reduced rates of TLR and target vessel revascularization (TVR) compared with ZES-E and/or first-generation PES. These findings therefore confirm on a larger-scale comparable efficacy of BP-DES and second-generation DES shown in the recent NEXT trial, powered for TLR as a primary endpoint [70]. Although not a new finding, in this analysis, all “limus”-eluting stents, with the exception of ZES-E, were associated with significantly lower rates of TLR and TVR than was the first-generation PES. This finding could derive from the differences in the healing process after implantation between paclitaxel and “limus”-eluting stents. Indeed, the toxicity caused by the long-lasting presence of paclitaxel in the vessel wall could give rise to vascular healing process, with prolonged fibrin deposition and inflammation, as shown in preclinical and postmortem studies [34, 73]. On the other hand, with ZES-E, short-release kinetics could result in insufficient inhibition of neointimal hyperplasia. Indeed, the more recently introduced Resolute ZES, which has a much longer (up to 180 days) release curve of the same antiproliferative agent, zotarolimus, is associated with a significant reduction in TLR and TVR compared with ZES-E.
There are in vitro data that raise issues with regard to biodegradable polymer technology. (1) It has been demonstrated that the polymer-based coating of the biodegradable stent (Biomatrix, Biosensors, Singapore) provides lower elasticity than durable polymers. This may lead to defects and fragility (cracks) of the coating following stent expansion during more than mild overstretches of the stent [77]. Therefore, after post-dilatation, embolization of material and microvascular obstruction could occur, or there might be reduced antiproliferative power because of detachment of polymer fragments. (2) The chronic swelling of the stent as it absorbs water to dissolve has been shown to influence the degree of neointimal hyperplasia [85]. Because the biodegradable polymer is expected to be totally degraded within 12 months following device implantation, the stent irregularities are unlikely to result in unfavorable clinical events. Thus, these data are only hypothesis generating and need to be confirmed in large-scale clinical trials with a long follow-up.
Several recently published or presented randomized clinical trials (RCTs) have performed head-to-head comparisons of the first-generation DES with the new BDS. The LEADERS [66] study with an all-comer design was the first head-to-head comparison of a stent platform eluting biolimus from a biodegradable polymer with a first-generation SES. At 9 months follow-up, the primary endpoint, a composite of death, MI, and TVR, occurred in 9.2 % of patients treated with BP-DES and 10.5 % of patients treated with DES, demonstrating the non-inferiority of BP-DES compared to the DES (p for non-inferiority = 0.003). Similarly, the ISAR-TEST-4 [86] with 2,600 enrolled patients compared a novel biodegradable polymer-based, rapamycin-eluting stent with two leading limus-based DES, the Cypher (using sirolimus) (Cordis, Johnson & Johnson, Warren, NJ, USA) and the Xience (using everolimus) (Abbott Laboratories, Abbott Park, IL, USA). At both 30 days and 12 months, BP-DES was significantly non-inferior (p = 0.005) to the “limus” DES for a composite endpoint that included both safety (cardiac death/MI) and clinical restenosis. Conversely, the COSTAR DES trial [87] found that the novel platform was not non-inferior to a Taxus DES (Boston Scientific, Natick, MA, USA). At 8 months, the incidence of MACE (11.0 % vs. 6.9 %, p < 0.005) and late loss (0.49 mm vs. 0.18 mm, p < 0.0001) was significantly higher with the COSTAR stent. In a recent meta-analysis [84], no clear advantage for BP-DES was shown as compared to DES in the rates of TLR and TVR, despite a significant 6-month reduction in ISLL observed among the BP-DES-treated patients (Fig. 24.6). ISLL is a frequently used parameter to quantify the degree of neointimal hyperplasia after coronary stenting [88]. A strong, direct, and significant association between ISLL and clinical impact, measured as number needed to treat to prevent one TLR, has also been demonstrated [89]. In the current meta-analysis, the lack of association between ISLL and TLR may be explained by the fact that the majority of the included RCTs were underpowered for low rates of binary events (e.g., TLR). On the other hand, the use of a continuous variable like ISLL allowed the efficacy of BP-DES versus DES to be compared without the need for extremely large patient populations. The finding of a decreased ISLL with BP-DES as compared to DES in that meta-analysis suggests a potential anti-restenotic efficacy of BP-DES; however, caution must be exercised in interpreting this result, which needs to be confirmed in future large RCTs with longer follow-up.
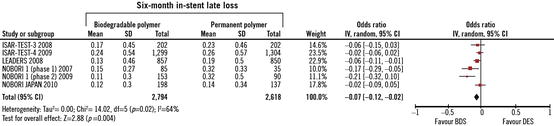

Fig. 24.6
A significant 6-month reduction in in-stent late loss (ISLL) observed among the patients treated with biodegradable polymer DES as compared to controls (Adapted from [84])
24.4.5 Polymer-Free DES
As a reflection of the dynamic progress in DES design, the new technology of biodegradable polymer DES and polymer-free DES has been developed and recently introduced to the market. Biodegradable polymer DES employs polymers that dissolve after time in which antiproliferative drug elution is needed. As shown in previous trials and a recent meta-analysis, first biodegradable polymer stents have been found not inferior to first-generation DES with long-term benefits seen mainly in regard to reduction of ST rates [66, 84]. On the other hand, once the degradation process of the polymer is completed in these devices, what remains is a bare-metal scaffold with thick-struts design. This platform may provide lower elasticity than durable polymers, with an increased risk of fragility and micro-damage to the coating, and potential “jailing” of side branches. As demonstrated in a landmark network meta-analysis, these factors explain the lower safety profile with biolimus biodegradable polymer stents as compared to the second-generation DES [32]. The improved design of polymer-free stents has addressed these limitations [90].
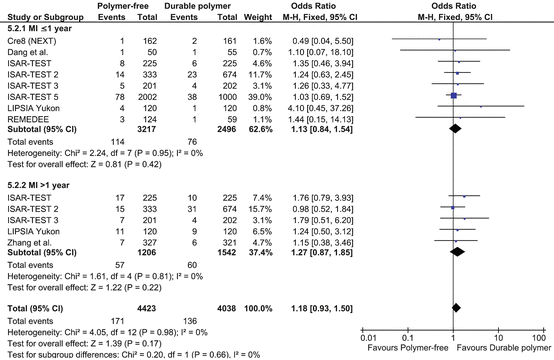
Clinical evidence from RCTs testing these new devices has however provided inconclusive results mainly due to the low number of enrolled patients. In the randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings—the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST) trial [91]—the polymer-free rapamycin-eluting stent was compared to durable polymer paclitaxel-eluting stents for the prevention of restenosis. No significant differences were observed in regard to 9-month angiographic and clinical outcomes. The combined incidence of death or MI was similar between the two groups (4.4 % in the rapamycin stent group vs. 4.0 % in the paclitaxel stent group) and restenosis-driven TLR rate was 9.3 % in both groups. The conclusions of the recently published 5-year follow-up of the ISAR-TEST trial [92] supported the hypothesis that the delayed ISLL reduction with polymer-free DES may indeed reduce the propensity for late inflammatory interactions, which in turn reduce rates of late thrombotic events. The incidence of ST, however, did not differ significantly between polymer-free DES and durable polymer DES arms in the trial (0.5 % vs. 1.6 %). The results of the present meta-analysis are in line with a recently published pooled analysis of individual patient data of 686 patients from two RCTs, ISAR-TEST, and LIPSIA Yukon trials [93]. However, the short follow-up analyzed the limited number of studies and patients included along with non-applicable publication bias analysis, hindered from drawing definitive conclusions with regard to the clinical performance of such devices. The recent meta-analysis [94] incorporated data of 6,178 patients with both short- and long-term follow-up, and its results were consistent in showing comparable but not superior outcomes with the polymer-free DES in comparison to their durable polymer counterpart in both the main and sensitivity analyses performed. Polymer-free technology has the potential advantage that might reduce the inflammatory and prothrombotic risk related to the polymer. On the other hand, these devices are basically BMS with some surface modification: either a roughened surface or microporous surfaces serving as reservoirs for drug; owing to the particular design of these devices, the modified process of coating through microporous surfaces or nanofilms might, however, impair the loading dose of the drug to be eluted [95].
Importantly, it has been shown that besides the efficacy and dose of a drug, drug release kinetics also has direct relevance with the treatment effect [96]. Drug release kinetics of polymer-free DES are likely to be different from those of permanent polymer DES due to the different strut surface; potential nonuniform drug deposition combined with the specially modified stent surface structures may lead to initially fast and later slow drug release [95]. Indeed, despite the advancement with nanoporous technology, the nanoporous polymer-free DES has brittle coating that may crack or delaminate during stent deployment, in turn resulting in a too rapid kinetic of drug release. A further attempt to improve the design of polymer-free DES has been made with polymer-free dual DES in the ISAR-5 trial [97], in which the antioxidant, probucol, has been added to rapamycin (sirolimus). Probucol, a lipophilic and antioxidant, may facilitate absorption of the drug into the vessel wall and drug retention in the vessel itself. However, it remains unclear whether the probucol’s known antioxidant action or its presumable role in facilitating a controlled sirolimus release was responsible for the improvements in stent performance seen in the trial. The recent reports support the thesis of polymer-free devices being comparable to durable polymer DES, without however providing additional benefits (Fig. 24.7). It should be noted that both short- and long-term adverse events were very low in the durable polymer group, even if the devices used were almost always first-generation stents. This finding probably reflects the period at which the included trials were performed, a time when the lessons of correct stent deployment and minimization of the risk of stent malapposition and under-expansion were well learned. Moreover, it should be well kept in mind that the mechanisms of ST are multifactorial and that the potential pro-inflammatory effect of polymers is only one of the leading factors. Thus, the efforts made by manufacturers in finding new solutions like the absence of a durable polymer should be praised but considered one of the many aspects of this late-occurring complication of PCI.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


