Chapter 64 Diseases of the Thoracic Cage and Respiratory Muscles
Respiratory Muscle Pump
The respiratory system is made up of two main components: the respiratory muscle pump, which facilitates airflow, thereby enabling ventilation, and the lungs, which support pulmonary gas exchange (see Chapter 6 for more detailed explanations and for respiratory muscle testing). The respiratory muscle pump itself is made up of the inspiratory muscles, the diaphragm and extradiaphragmatic accessory muscles, and the expiratory muscles, principally the abdominal wall muscles. Inspiration is an active process in which contraction of the diaphragm forces the abdominal contents in an anterocaudal direction to allow the volume of the chest cavity to increase. This expansion, in turn, generates a negative subatmospheric pressure to form a pressure gradient that drives air into the lungs. At rest, expiration is a passive process with the elastic properties of the chest wall and lungs providing the recoil forces that allow the lung volume to return to the functional residual capacity. During exercise and forced expiratory maneuvers (e.g., coughing), however, contraction of the abdominal muscles occurs, pushing the diaphragm upward in the absence of flow limitation.
Clinical Consequences of Respiratory Muscle Pump Imbalance
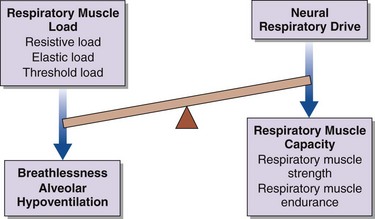
Breathlessness, alveolar hypoventilation and hypercapnic respiratory failure result from an imbalance between the respiratory muscle load, capacity, and neural respiratory drive (Figure 64-1). By using this model the physician can develop a simple clinical tool to assess the breathless patient and the patient in chronic respiratory failure. More commonly, in patients with restrictive lung disease, the reduction in capacity occurs as a consequence of intrinsic muscle weakness. On occasion, however, capacity may be relatively preserved, but in the context of a significant respiratory muscle load or poor orientation of the inspiratory muscles (e.g., kyphoscoliosis), this may limit the pressure-generating capacity, so that dyspnea and alveolar hypoventilation can develop subsequently. In critically ill patients, the respiratory muscle weakness may be transient and reversible, such as occurs in respiratory acidosis, hypokalemia, and hypophosphatemia (Box 64-1).
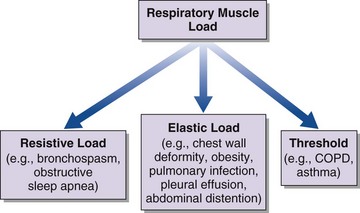
An increase in respiratory muscle load occurs in patients with increased airway resistance, chest wall deformity, or obesity. Any of these conditions will accelerate the onset of dyspnea and alveolar hypoventilation in patients with respiratory muscle weakness (Figure 64-2).
Assessment of the Respiratory Muscle Pump
Clinical Features
As always, a detailed history will facilitate making a correct diagnosis (Table 64-1). Of particular importance is the rate of onset of symptoms. Acute respiratory muscle weakness tends to progress rapidly, culminating in a medical emergency requiring intubation and mechanical ventilation. Some causes of acute respiratory muscle weakness, such as an acute myasthenic crisis or Guillain-Barré disease, may be reversible, and it is important to facilitate invasive respiratory support in the acute setting until a diagnosis can be made and appropriate therapies initiated. In chronic respiratory failure, the onset may not be clear, but clues to the course of events can be gained from the history. Often patients will describe dyspnea on exertion, but if peripheral muscle weakness precedes respiratory muscle weakness, the consequent loss of significant locomotor function will not permit exertion of sufficient degree to produce dyspnea. Classic features of diaphragm weakness are not always present in patients with generalized neuromuscular disease but may include dyspnea on lying supine, on bending forward, or on immersion in water above the midchest level. If respiratory weakness is severe, patients also may describe symptoms of nocturnal hypoventilation, such as extensive daytime somnolence, reduced concentration, and morning headaches that typically resolve within 30 minutes of waking. Specifically, in the pediatric and adolescent populations, clinicians should be alerted to more subtle clinical features such as failing school performance, recurrent episodes of chest sepsis, reduction in appetite, and weight loss.
Table 64-1 Important Clinical Features in Diseases of the Thoracic Cage and Respiratory Muscles
| Disorder | Clinical Manifestations |
|---|---|
| Sleep-disordered breathing | Morning headache, daytime sleepiness, disrupted sleep pattern, impaired intellectual function, generalized fatigue, loss of appetite and weight |
| Respiratory muscle weakness | Orthopnea, breathlessness on immersion in water, breathlessness on leaning forward, breathlessness on exertion, poor cough, poor chest expansion, paradoxical abdominal motion during inspiration (inward motion of the anterior abdominal wall due to diaphragm weakness), abdominal muscle recruitment in expiration |
| Bulbar dysfunction | Low volume voice, difficulty swallowing, drooling, difficulty clearing secretions, poor cough, staccato/slurred speech, coughing on swallowing |
Pulmonary Function Tests
The pattern of respiratory muscle weakness must be considered in interpreting results of pulmonary function tests in patients with chest wall disease and respiratory muscle weakness. Although total lung capacity (TLC) and VC are reduced in patients with predominantly inspiratory muscle weakness and combined inspiratory and expiratory muscle weakness, the pattern of weakness influences functional residual capacity (FRC), residual volume (RV), overall gas transfer (DLCO), and gas transfer corrected for alveolar volume (Table 64-2).
Table 64-2 Pulmonary Function Tests for Patients with Predominantly Inspiratory Muscle Weakness and Combined Inspiratory/Expiratory Muscle Weakness
| Inspiratory Muscle Weakness | Combined Inspiratory/Expiratory Muscle Weakness |
|---|---|
| Reduced VC | Reduced VC |
| Fall in supine VC >20% | N/A |
| FEV1/FVC ratio >80% | FEV1/FVC ratio >80% |
| Reduced TLC | Reduced TLC |
| Normal RV | Increased RV |
| Reduced FRC | Increased FRC |
| Reduced TLCO | Reduced TLCO |
| “Supranormal” KCO | Reduced KCO |
FEV1, forced expiratory volume in 1 second FRC, functional residual capacity; FVC, forced vital capacity; KCO, gas transfer coefficient corrected for alveolar volume; N/A, not available; RV, residual volume; TLC, total lung capacity; TLCO, overall gas transfer; VC, vital capacity.
Tests of Respiratory Muscle Function
Because respiratory muscle contraction generates tension, the consequent pressure change that occurs can serve as an in vivo measure for the quantification of respiratory muscle strength. In addition, recent developments have expanded the current understanding of the mechanical actions and interactions of the respiratory muscles. A greater emphasis has been placed on assessing and quantifying respiratory muscle strength and pulmonary mechanics in a variety of patient groups, including children, adolescents, and adults (see Chapter 6 for a more detailed discussion).
Sniff Inspiratory Pressure
The rapid inspiratory effort of a sniff maneuver (see Chapter 6) is accompanied by momentary equilibration of intrathoracic and upper airway pressures. This equilibration occurs above a pressure of 10 to 12 cm H2O, so employing sniff nasal pressure (Pnsn) allows noninvasive measurement of inspiratory muscle strength. Pnsn is a particularly useful additional investigation in patients with a low or equivocal PImax to confirm or exclude the presence of inspiratory muscle weakness.
Maximum Transdiaphragmatic Pressure Measurements
The previously described tests provide data only on global respiratory muscle function, rather than on diaphragm function itself. To specifically assess diaphragm function, transdiaphragmatic pressure (Pdi) is measured by placement of a pressure-monitoring catheter in both the esophagus and stomach, to provide measurement of the esophageal and gastric pressures (Figure 64-3).
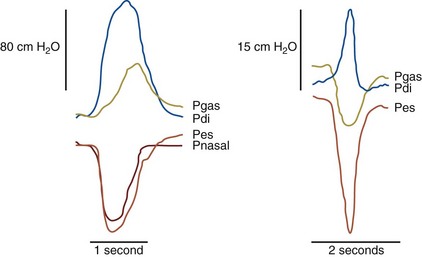
Although transdiaphragmatic pressure during a maximal static maneuver (Pdimax) has been reported, this maneuver gives a wide normal range in naive (i.e., previously untested) subjects. An alternative is to obtain maximal sniff transdiaphragmatic pressure (Pdisn), which has gained recognition in clinical practice not only because it is a familiar maneuver but because it measures both Pessn and Pdisn, providing data on global inspiratory muscle and diaphragm strength (Figure 64-4).