11 OBJECTIVES
Diseases of the Pleura
 Review the anatomy and histology of the pleura.
Review the anatomy and histology of the pleura.
 Review the physiology of pleural fluid formation and absorption.
Review the physiology of pleural fluid formation and absorption.
 Discuss the diagnosis and pathophysiology of pleural effusions.
Discuss the diagnosis and pathophysiology of pleural effusions.
 Differentiate pleural effusions into transudates and exudates.
Differentiate pleural effusions into transudates and exudates.
 Review the diagnosis, pathogenesis, and pathophysiology of pneumothorax.
Review the diagnosis, pathogenesis, and pathophysiology of pneumothorax.
GENERAL CONSIDERATIONS
The term pleura refers to the membranous covering that overlies the organs within the thoracic cavity. It is divided into the nonpulmonary parietal and the pulmonary visceral pleura. Within the 10–20-μm distance between the pleural surfaces is the pleural fluid—a low-protein ultrafiltrate derived from the pleural micro-circulation. The pleura anatomically and physiologically couples the lungs to the chest wall; furthermore, it has a significant impact on respiratory physiology during health and in illness.
Anatomy & Histology
The parietal pleura covers the chest wall (costal pleura), diaphragm (diaphragmatic pleura), and mediastinum (mediastinal pleura). The visceral pleura covers the lung parenchyma and lobar fissures. The pleura is made up of two layers: mesothelium and connective tissue. Mesothelial cells are of variable sizes (6–12 μm) and contain microvilli on their surface. The microvilli are present over the entire pleural surface, but their density varies depending on their location. Although the function of the microvilli is not completely certain, it appears that they enmesh glycoproteins (such as hyaluronic acid) to reduce friction between the parietal and visceral surfaces. It is estimated that 0.1–0.2 mL/kg of low-protein fluid is normally present between layers. The cells present in normal pleural fluid are mainly macrophages, monocytes, and mesothelial cells, although lymphocytes and neutrophils may also be present. Pleural fluid typically contains fewer than 1000 cells/mL.
The connective tissue layer of the visceral pleura contributes to the elastic recoil of the lung. It is composed of a network of collagen and elastin that prevents hyperinflation of the lung. Since stomata are not present in the connective tissue layer of the visceral pleura, fluid reabsorption does not occur there. The micro-circulation contained within this layer is supplied by the bronchial artery and drained via the pulmonary vein. Pleuritic pain indicates injury or inflammation of the parietal pleura as there is no innervation of the visceral pleura.
Pleural Physiology
PLEURAL FLUID FORMATION
In the healthy state, pleural fluid is an ultrafiltrate that originates from the micro-vasculature of both the visceral and the parietal pleura. Fluid enters the pleural space due to a pressure gradient that exists between the pleural interstitium and the pleural space at a rate of 0.01 mL/kg/h.
Pleural fluid formation is thought to be determined by Starling’s hypothesis of transcapillary exchange:
Qf = Lp* A [(Pcap − Ppl) − σd (πcap − πpl)]
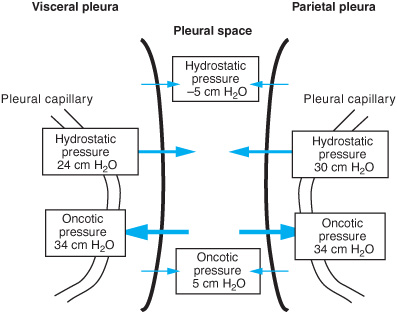
where Qf = liquid movement, Lp = filtration coefficient, A = surface area; P = hydro-static pressure in the capillary (cap) or pleural space (pl), π = oncotic pressure in the capillary (cap) or pleural space (pl), and σd solute reflection coefficient (the membrane’s resistance to passage of large molecules). Figure 11–1 shows the various pressures involved in the movement of fluid.
Therefore, formation of pleural fluid is determined by the hydrostatic pressure gradient (vascular minus pleural) minus the oncotic pressure gradient (vascular minus pleural).
As depicted, the net balance of forces in the visceral pleura is zero. It is hypothesized that there is little contribution from the visceral pleura to the development of pleural fluid; however, this has not definitively been demonstrated in human subjects. The filtration coefficient for the visceral pleura is less than that for the parietal pleura due to the greater distance between the visceral pleural capillaries and the pleural space. In the parietal pleura, the net balance favors pleural fluid formation.
Figure 11–1. Pressures influencing pleural fluid formation. The net pressure across the visceral pleura is zero, whereas the net pressure across the parietal pleura is approximately 6 cm H2O. Thus, the capillaries of the parietal pleura are the major source of pleural fluid formation. However, under certain conditions more fluid may come from the interstitium of the lungs across the visceral pleura.
THE PLEURA IN DISEASE STATES
Pleural Effusions
PATHOGENESIS
In the normal state, 0.5 mL of pleural fluid is formed every hour in human beings. To maintain a constant volume of fluid in the pleural space, fluid must be resorbed at the same rate it is formed. The parietal pleural lymphatics provide the pathway for drainage of the pleural fluid. Thus, formation of a pleural effusion results either from overproduction of pleural fluid or from inability of the lymphatic system to remove fluid as it is produced. Pleural fluid accumulates when intrapleural fluid loads are 30 times the normal steady state.
Pleural effusions due to increased fluid formation occur in the following settings:
1. increased interstitial fluid production (congestive heart failure, renal failure, pneumonia, or iatrogenic volume overload);
2. increased hydrostatic pressure in the pleural capillaries (left ventricular failure, pericardial effusion, or superior vena cava obstruction);
3. increased capillary permeability (pleural inflammation);
4. excessive peritoneal fluid (ascites or peritoneal dialysis in the presence of a diaphragmatic defect);
5. disruption of thoracic blood vessels or the thoracic duct (surgery, trauma, or malignancy);
6. decreased pleural pressure (bronchial obstruction).
Pleural effusions due to decreased pleural fluid absorption occur in the following settings:
1. elevation of systemic venous pressures (intravascular volume overload from any cause);
2. compression or dysfunction of pulmonary lymphatics (malignancy, lymphadenopathy, or a primary lymphatic disorder).
PATHOPHYSIOLOGY
The formation of pleural fluid can affect all organ systems adjacent to the pleura, especially the heart and lungs. The size of a pleural effusion generally correlates with the degree of physiologic abnormality, but is not always accompanied by dyspnea. The symptom of dyspnea is associated with a pleural effusion when there is underlying parenchymal lung disease, chest pain, splinting, or diaphragmatic dysfunction.
As pleural fluid accumulates, the shape and function of the diaphragm change. Initially the diaphragm remains domed and functions normally. However, with further increases in pleural fluid volume, the diaphragm becomes flattened and fixed. Continued accumulation eventually results in inversion of the diaphragm and subsequent dysfunction. These effects are more predominant on the left side of the chest because the presence of the liver on the right side prevents inversion.
Significant pleural effusions impose a restrictive ventilatory defect on the lungs. In animal and human models, a pleural effusion decreases total lung capacity and forced vital capacity by only 20%–30% of the effusion’s size, so the remaining volume of the effusion must increase the size of the thoracic cavity. It is thought that this increase in cavity size contributes significantly to the sensation of dyspnea. Following thoracentesis, the length–tension relationship of the inspiratory muscles improves to create a more negative intrapleural pressure at any given lung volume.
In addition to affecting the lung and thoracic cavity, large pleural effusions can also alter cardiac function. In animal and human studies, the right and left ventricles have been reported to collapse as a result of pleural pressure elevation. The pressure elevation and cardiac collapse are reversible with therapeutic thoracentesis.
DIAGNOSTIC CONSIDERATIONS
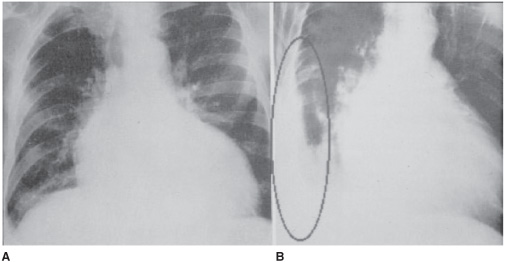
Pleural effusions may initially be discovered by physical exam. Findings include absence of breath sounds on auscultation, decreased tactile fremitus, and dullness to percussion. Pleural effusions can then be confirmed by chest radiography. If a patient has a freely flowing effusion, it will settle into the most gravity-dependent portion of the chest. Thus the location and appearance will vary depending on the position of the patient and the amount of fluid present. For unclear reasons pleural fluid can accumulate between the inferior surface of the lung and the superior surface of the diaphragm (referred to as a subpulmonic effusion). If the fluid remains in this position it results in the appearance of an elevated or abnormally shaped hemidiaphragm (Figure 11–2). A left subpulmonic effusion may be seen as a large (>2 cm) separation between the gastric bubble and the hemidiaphragm.
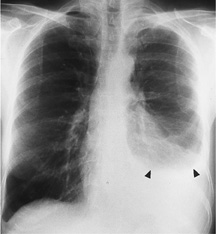
When more than 75 mL accumulates, pleural fluid usually spills into the costophrenic sulcus resulting in blunting of the costophrenic angle on radiograph. It is the presence of fluid in this location that accounts for the meniscus sign that is the classic sign of a pleural effusion (Figure 11–3).
For patients in the supine position, pleural effusions can be especially difficult to diagnose as the meniscus sign is often absent. Fluid tends to layer posteriorly in the supine position and appears as a general opacification of the hemithorax. Pleural fluid opacifications are without air bronchograms and the pulmonary vasculature is visualized.
Some effusions are not free flowing and are referred to as loculated. This may result from inflammation or scarring of the pleural surface. Occasionally fluid can be trapped in an interlobar fissure, causing a mass-like opacity or pseudotumor (Figure 11–4).
Despite their widespread use to visualize pleural effusions, chest radiographs are relatively insensitive in determining pleural fluid accumulation. Computed tomography, on the other hand, is extremely sensitive and can determine the presence of as little as 2–10 mL of fluid (Figure 11–5).
Ultrasonography is becoming an important imaging tool for diagnosing pleural effusions. It is inexpensive and can be performed at the bedside to locate and quantify effusion. Ultrasound can help rule out other etiologies of radiographic opacification including atelectasis, consolidation, mass or elevated hemidiaphragm. This is especially the case for critically ill patients who may be more difficult to move. Diagnostic thoracentesis should be performed simultaneously and ultrasound guidance results in fewer complications.
Figure 11–2.A. Subpulmonic effusion. The right hemidiaphragm is elevated and its apex displaced medially. B. Here the subpulmonic effusion is confirmed by a lateral decubitus view.
Figure 11–3. A free-flowing pleural effusion. This image shows a large left pleural effusion obliterating the heart border and hemidiaphragm. A meniscus can be seen in this upright x-ray (arrowheads). There is also a small effusion on the right, as manifested by blunting of the right costophrenic angle.
EVALUATING PLEURAL FLUID
Once a pleural effusion is recognized, a decision must be made regarding further evaluation of the fluid. Diagnostic examination is performed by a thoracentesis. This procedure should be performed with any clinically significant pleural effusion (at least 10 mm on a lateral decubitus film) that is unexplained. Effusions associated with fever, pain, or other atypical characteristics should be evaluated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree