Diseases of the Great Vessels: Aorta and Pulmonary Artery
Anupama Rao
High-Yield Findings: Aorta and Pulmonary Artery
Aortic root measurements are made in a modified parasternal long-axis view at end-diastole.
It is important to index aortic size for body surface area. Indications for surgical correction are based on the underlying condition.
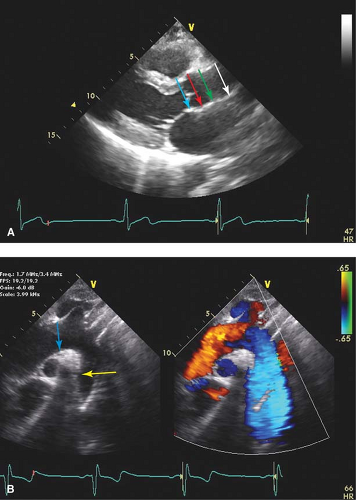
With aortic dissection, determine where flow in systole is occurring—this usually identifies the true lumen.
Intramural hematomas are treated similarly to aortic dissection.
McConnell’s sign in combination with 60/60 sign is specific for pulmonary emboli.
Key TTE Views: Aorta and Pulmonary Artery
Standard parasternal long axis: Descending aorta in cross-section
Modified parasternal long axis (probe is placed in a superior rib space than standard PLAX): Aortic valve annulus, sinuses of Valsalva, sinotubular junction, ascending aorta
Right ventricular outflow view: Main pulmonary artery
PSAX: Aortic valve leaflets, sinuses of Valsalva, right ventricular outflow tract, and main pulmonary artery
Off-axis A4C and A2C (posterior tilt): Descending aorta
Subcostal view (long axis): Proximal abdominal aorta
Suprasternal notch: Aortic arch, identification of branch vessels: Inominate, carotid and subclavian arteries, descending aorta, right pulmonary artery
While transthoracic echocardiography (TTE) provides useful serial measurement of the great vessels, transesophageal echocardiography (TEE) is the ultrasound modality of choice in comprehensively imaging these structures, especially in emergency situations.
Aorta
Anatomy
The aorta is divided into the thoracic and abdominal aorta.
The thoracic aorta is further divided into the aortic root, arch and ascending and descending aorta.
The aortic root consists of the aortic annulus, the sinuses of Valsalva and the sinotubular junction, which joins the proximal ascending portion of the thoracic aorta (Fig. 16-1).
The ascending portion continues until the origin of the inominate artery.
The aortic arch gives rise to the inominate, left main carotid, and left subclavian arteries.
The descending aorta begins after the origin of the left subclavian artery and continues past the diaphragm becoming the abdominal aorta (Fig. 16-2). The ligamentum arteriosum is just distal to the left subclavian artery, anchoring the descending aorta to the thorax.
Key Point:
This area in between the ligamentum arteriosum and the left subclavian artery is known as the aortic isthmus, which is the most common location for abnormalities such as coarctation, patent ductus arteriosus, and dissection due to trauma or deceleration injury.
TEE is an excellent modality to image the entire aorta apart from a small “blind-spot” where the air-filled trachea shields a segment of the distal ascending aorta as it becomes the proximal aortic arch.
Key TEE Views
Upper esophageal (20 to 25 cm) 0-degree long axis: Aortic arch
Upper esophageal (20 to 25 cm) 90-degree short axis: Aortic arch along with pulmonary artery
Mid-esophageal (30 to 40 cm) 30- to 40-degree short axis: Aortic valve
Mid-esophageal (30 to 40 cm) 100- to 120-degree left ventricular long-axis view: Aortic valve and root
Deep transgastric view (45 to 50 cm) 0- to 20-degree flexion: Aortic valve and root
Turning probe posteriorly: Transgastric to upper esophageal views 0- and 90-degree views of thoracic aorta
Pathology
Aortic Aneurysm
An aortic aneurysm is defined as the presence of dilatation of over 50% of the normal diameter (>2.75 cm/m2), involving all three layers of the vessel (intima, media, and adventitia). Etiologies include hypertension, atherosclerosis, poststenotic dilation, cystic medial necrosis, collagen vascular diseases such as Marfan’s syndrome and systemic lupus erythematosus as well as inflammatory states such as rheumatoid arthritis and Reiter’s syndrome.
Key Point:
Patients with Marfan’s syndrome usually have a pattern of aortic dilatation, which starts in the sinuses of Valsalva and expands distally (“pear”-shaped aortic root) (Fig. 16-3).
Measurements of aortic diameter are usually made in the modified parasternal long-axis view at end-diastole. Serial annual echocardiographic measurements are made once aortic dilatation (upper normal limit is 2.1 cm/m2 at aortic sinuses) is detected.
Measurements include:
Aortic annulus
Sinuses of Valsalva
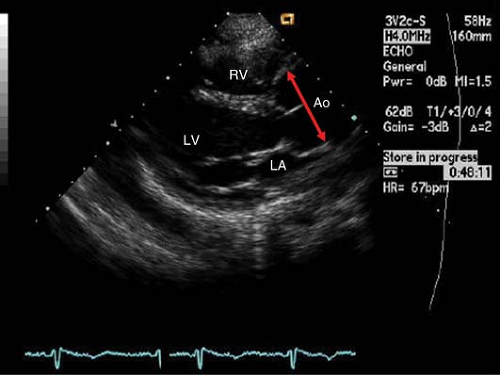
Figure 16-3. PLAX view showing aortic root dilatation at the sinuses of Valsalva (double-headed arrow) in a patient with Marfan’s syndrome.
Sinotubular junction
Proximal ascending aorta
Aortic arch (suprasternal view)
Abdominal aorta (subcostal view)
Indications for Operative Intervention for Thoracic Aneurysms
Ascending aorta or aortic sinus diameter ≥5.5 cm
Genetically mediated disorders with higher risk of dissection (e.g., Marfans, Ehlers Danlos, Bicuspid aortic valve, Turner’s syndrome, Loeys–Dietz syndrome, Familial thoracic aortic aneurysm or dissection) ≥4–5 cm depending on condition
Ascending aorta or aortic root >4.5 cm in patients already undergoing aortic valve replacement
Rapidly expanding aneurysms (>5 mm/year) when ascending aorta or aortic sinus <5.5 cm
Sinus of Valsalva Aneurysm
These aneurysms usually involve abnormal dilatation of one of the sinuses of Valsalva (Fig. 16-4).
Sinus of Valsalva aneurysms (SVA) may be congenital or due to processes that involve the aortic root such as Marfan’s syndrome, syphilis, endocarditis, trauma, or prior surgery.
SVA may appear as a “windsock” deformity projecting into adjacent structures.
SVAs originate most commonly from the right coronary sinus.
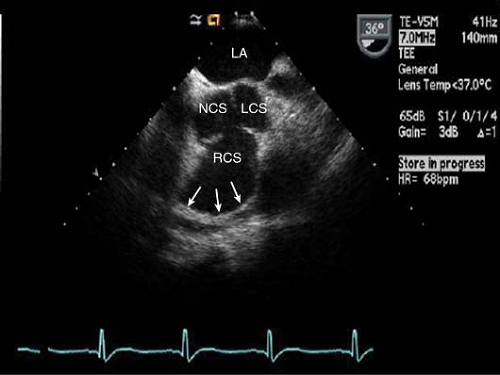
Figure 16-4. Short-axis TEE image of the aortic valve demonstrating a large right coronary sinus of Valsalva aneurysm (NCS, non-coronary sinus; LCS, left coronary sinus; RCS, right coronary sinus).
Aneurysms of the right coronary sinus protrude into the RV outflow tract, while left coronary sinus aneurysms protrude into the left atrium and the non-coronary sinus into the right atrium.
Associated conditions include ventricular septal defect (VSD), bicuspid aortic valve, aortic regurgitation, pulmonary stenosis, atrial septal defect (ASD), and coarctation of the aorta.
Key Point:
Rupture of SVA into adjacent structures is the main cause of mortality. Cardiac tamponade can occur if rupture occurs into the pericardial sac.