AORTA/PERIPHERAL VASCULAR DISEASE
CHAPTER
Diseases of the Aorta
Diseases of the aorta account for significant cardio-vascular morbidity and mortality. The incidence of these diseases is expected to rise with the increasing age of the population. Diagnostic evaluation of aortic disorders has vastly improved over the last two decades, allowing for earlier diagnosis and therapeutic intervention. This chapter summarizes the major disease entities affecting the aorta.
ANATOMY OF THE AORTA
The aorta is the main conduit and reservoir of blood in the body. It is an elastic artery composed of three layers:
1. The intima, which includes the single-layered endothelium
2. The media, which is the thickest layer of the aortic wall. It is composed of sheets of elastic tissue and collagen, which provide the aorta its tensile strength and distensibility. Smooth muscle cells and ground substance are also present. The components of the media are organized into functional units known as lamellae.
3. The adventitia, which is composed of loose connective tissue and contains nerves and the vasa vasorum, which provides the main blood supply to the aortic wall. Elastin, collagen, and fibroblasts are also present.
Anatomically, the aorta is divided into two main subcomponents:
1. The thoracic aorta consists of the aortic root (from the aortic annulus, including the sinuses of Valsalva, up to the level just above the sinotubular junction), the ascending aorta (average diameter 3 cm), the arch, and the descending thoracic aorta (average diameter 2.5 cm—begins after the origin of the left subclavian artery).
2. The abdominal aorta, which is the part of the descending aorta after it passes through the diaphragm. The abdominal aorta (average diameter 2.0 cm) is further classified as either suprarenal or infrarenal.
PATHOLOGIC PROCESSES
Cystic Medial Degeneration
Cystic medial degeneration is an important predisposing factor to diseases of the aorta, particularly the ascending aorta. It is characterized by smooth muscle cell loss and apoptosis plus fragmentation of elastic fibers within the media of the aortic wall. Basophilic ground substance occupies these areas of structural defects, which are incorrectly termed cysts. This degenerative process also extends to the elastic components of the adventitial layer. The weakened aortic wall is prone to aneurysm formation and dissection. This degenerative process, which may be determined genetically, is seen classically in connective tissue diseases such as Marfan, Loeys-Dietz, and Ehlers–Danlos syndrome. However, various degrees of degeneration can be seen in patients without these disorders, occurring as an idiopathic variant, in familial syndromes, or as an acquired form. Hypertension and advancing age are associated with the latter. Varying degrees of cystic medial degeneration can also be seen in genetically predisposed aortas in association with congenital abnormalities including bicuspid or unicuspid aortic valve, aortic coarctation, Turner syndrome, and Noonan syndrome.
Atherosclerosis
Atherosclerosis appears to play a significant role in diseases of the aortic arch, descending thoracic and abdominal aorta. Atherosclerosis can result in weakening of the aortic wall, making it prone to aneurysm formation or dissection.
The development of aortic atherosclerosis is associated with the traditional cardiac risk factors of smoking, hypertension, hyperglycemia, and atherogenic lipoproteins. Atherosclerosis can also result in formation of complex atheromatous plaques, which are prone to embolization, resulting in cerebral and peripheral arterial events.
Inflammatory Disorders
Inflammatory disorders represent a third broad category in the etiology of aortic diseases. These can occur in isolation or in the context of systemic disorders.
Trauma
Aortic injury from trauma usually occurs as a result of deceleration injuries. It frequently occurs at the level of the left subclavian artery near the aortic isthmus or near the diaphragm, both points of attachment. If the patient survives, injury can progress to form a chronic pseudoaneurysm.
AORTIC DISSECTION
Aortic dissection comprises one of the more ominous acute aortic syndromes (also known as acute thoracic pain syndromes), which include the dissection variants of penetrating aortic ulcers, intramural hematomas, and symptomatic aneurysms. It involves cleaving of the aortic wall, resulting in the formation of an aortic false lumen, which courses along with a true lumen.
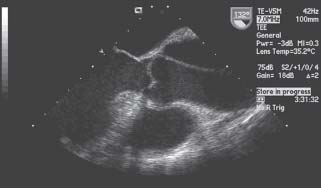
The hallmark of aortic dissection is an intimal tear that permits access of pulsatile high-pressure blood into the aortic media, separating it from the basal layers (Fig. 48.1). Typically, the so-called intimal flap is usually an intimal–medial flap.
FIGURE 48.1 TEE in a long-axis view that shows a large dissection flap in the ascending aorta extending from the level of the aortic sinuses.
The initiating event of dissection may be a tear in the intima. Alternatively, primary rupture of the vasa vasorum may result in an intramural hematoma that leads secondarily to an intimal tear as blood vents from the intramural space (Fig. 48.2). Regardless of the initiating event, the force of blood flow propagates the dissection antegrade (and less commonly retrograde), a variable extent along the vessel, cleaving the aortic wall usually along the outer one-third of the medial layer.
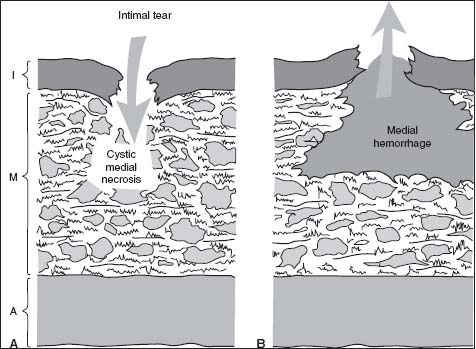
FIGURE 48.2 Schematic representing the initiating event of aortic dissection. Panel (A) shows a tear in the intima. Panel (B) shows primary rupture of the vasa vasorum, secondarily leading to an intimal tear as blood vents from the intramural space.
Classification
Dissections are classified by their location of origin and how far along they extend in the aorta. There are two important classification systems: the DeBakey system and the Stanford system (Table 48.1; Fig. 48.3). Dissections are also classified by their duration. Acute dissections are those of <2 weeks’ duration after symptom onset; chronic are those that have been present for more than 2 weeks.
TABLE
48.1 Classification of Aortic Dissections
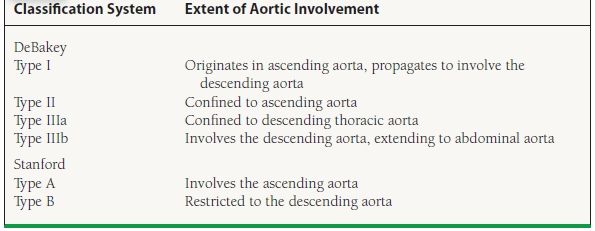
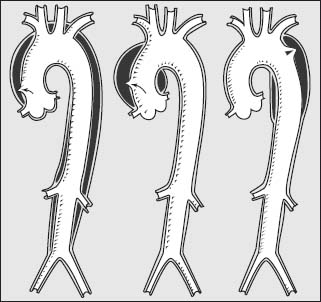
FIGURE 48.3 Diagram showing the types of aortic dissection by the DeBakey classification. Shown are types I, II, and IIIa, from left to right.
Dissections typically present between the fifth and seventh decades of life, with a male preponderance. Patients typically present with the acute onset of pain, present in up to 95% of cases. Pain is often most severe at its onset and described as a “ tearing,” “ ripping,” or “ stabbing” sensation. Often the pain is migratory, a crucial component of the history, reflecting propagation of the dissection. Involvement of the ascending aorta results in anterior chest or neck pain, with intra-/subscapular pain with involvement of the descending thoracic aorta, and lower back and left flank pain with the thoracoabdominal aorta.
Hypertension upon presentation is common, more so in distal dissection, although hypotension can be seen if complications have developed, particularly in proximal dissections. The dissection may compromise flow to the great vessels, and pulse deficits (these can be transient, as the dissection flap can oscillate) may be present. Actual blood pressure may not be appreciated if the arm utilized has compromise of the brachial vasculature (pseudohypotension).
If the dissection involves the aortic root, commissural involvement of the aortic valve can lead to aortic insufficiency. Dilatation of the root and aortic annulus, without leaflet involvement, can also lead to aortic valve insufficiency. A diastolic murmur will be evident in these cases.
Dissections can involve the ostia of the coronary arteries, resulting in acute myocardial ischemia and infarction (present in 2% to 3% of cases). The right coronary artery ostium is more commonly affected than the left main. The dissection can extend proximally into the pericardial space, resulting in pericardial effusion and tamponade, a common mechanism of syncope and hypotension in dissection. A pericardial friction rub can be a clue to the presence of hemopericardium. Rupture into the pericardial space represents the most common mode of death in patients with aortic dissection. Acute lower-extremity, renal, or mesenteric ischemia can be seen in descending aortic dissections. Focal neurologic deficits can occur with involvement of the great vessels. Compromise of spinal artery perfusion may result in paraparesis.
Although chest pain and pulse deficits are classically described, it is important to recognize that <20% of patients present with these findings. Therefore, a high clinical suspicion for dissection is paramount.
Diagnostic Testing
The chest x-ray can be normal in cases of dissection. A well-recognized finding is mediastinal widening, present in about 60% of cases. Rupture into the pleural or pericardial space manifests as pleural effusions or an enlarged cardiac silhouette (the latter may also be present, as a result of chronic aortic insufficiency). The electrocardiogram may be normal, but it often shows nonspecific ST–T-wave changes. Involvement of the coronary artery ostia may result in ST-segment elevation, representing an acute myocardial injury pattern. Transthoracic echocardiography can on occasion identify a proximal or even distal dissection flap. Even if a flap is not seen, the presence of aortic dilatation, aortic insufficiency, and/or an unexplained pericardial effusion can be important clues in the diagnostic consideration of a patient with chest pain.
More definitive diagnostic modalities include transesophageal echocardiography (TEE), computed tomography (CT), and magnetic resonance angiography (MRA). Each has relative advantages and disadvantages, but all have excellent sensitivity and specificity (Table 48.2).
TABLE
48.2 Comparison of Imaging Modalities for Aortic Dissection
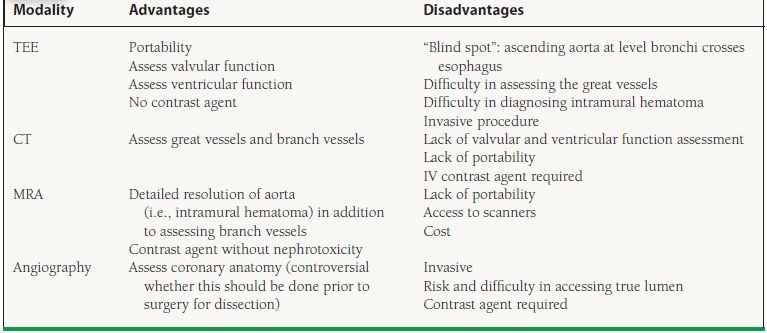
TEE, transesophageal echocardiography; CT, computed tomography; MRA, magnetic resonance angiography.
Angiography is less commonly utilized for the primary diagnosis of aortic dissection. The test of choice is often dependent on expedited availability and expertise at the center where the patient is evaluated. An important caveat is that in most patients, more than one test may be required. If the clinical suspicion is high enough and the initial test is negative or equivocal, then consideration should be given to performing another confirmatory test.
Management
Anti-impulse medical therapy should be initiated as soon as the diagnosis of dissection is considered, even while waiting confirmatory diagnostic testing. In patients who are hypertensive, intravenous beta-blockade followed by sodium nitroprusside is the treatment of choice. Beta-blockade should be initiated prior to sodium nitroprusside to avoid a rise in cardiac contractility and dp/dt associated with the isolated use of vasodilators. In the absence of hypertension, beta-blockers can be used alone. For patients with ascending aortic dissections, these are temporizing agents while preparing for definitive surgical therapy. For patients with descending dissections, these agents are first-line therapy, before longer-acting oral agents are initiated. Intravenous nondihydropyridine calcium antagonists such as verapamil and diltiazem are alternatives for those who cannot tolerate beta-blockers.
Dissections that involve the ascending aorta (proximal, type A) require urgent surgical therapy, as there is a very high early mortality rate (approaching 1% to 2% per hour for the first 24 to 48 hours). Even with timely surgical intervention, the mortality rate of proximal aortic dissection approaches 25%.
An important management point arises with patients who have pericardial effusion or tamponade in association with a proximal dissection. These patients should not undergo percutaneous pericardiocentesis, unless they are in absolute extremis. The evacuation of pericardial blood by such a route has been associated with aortic rupture and increased mortality, perhaps secondary to dissection extension and/or aortic rupture as blood pressure and dp/dt increase after tamponade resolution. Pericardial access should be obtained in the operating room, with the institution of cardiopulmonary bypass.
Dissections that involve the descending aorta (distal, type B) should be initially treated medically. Data suggest that medical therapy is the preferred initial treatment, with surgery guided by a complication-specific approach. This is because acute aortic surgery is associated with a high mortality and paraplegia rate (inadequate protection of the spinal arteries). Surgery should be considered for the following indications: evidence of malperfusion syndromes (organ ischemia secondary to compromise of the branch vessels); persistent pain; aneurysm formation, particularly if saccular; and retrograde dissection to a proximal extent. Alternatively, aortic fenestration, surgical or percutaneous, can also be considered for organ or limb malperfusion in carefully selected patients.
Distal (type B) dissections in Marfan syndrome patients carry a poor prognosis and have thus led to recommendations of early aortic surgery.
Aortic Dissection in the Young
Dissections occurring in younger patients (<40 years old) typically occur in the context of connective tissue disorders such as Loeys-Dietz, Marfan, or Ehlers–Danlos syndrome. Other conditions involving younger patients and dissection include congenital bicuspid aortic valve, patients with prior aortic surgery, or women in the peripartum period. During late pregnancy, it is thought that hormonal changes and a loosening in the ground substance of connective tissue can predispose to a heightened risk of dissection.
Chronic Aortic Dissection
Chronic dissection patients (present for >2 weeks) have survived the period of increased mortality. They can often be managed medically, even in the presence of a proximal dissection. However, their aortas often dilate and are at higher risk for aneurysm formation because of the thinned aortic wall as a result of dissection.
A complication-specific approach can be used for chronic dissection patients to guide elective surgical therapy: recurrent pain; aneurysm formation, particularly if saccular; and retrograde dissection extension to a proximal extent. Serial follow-up imaging (usually with CT or MRA), initially at shorter intervals, is vital in these patients because of their weakened aortic walls.
Iatrogenic Aortic Dissection
Special mention should be afforded to iatrogenic dissections. Angiographic catheters and guidewires can disrupt the intima and result in dissections anywhere along the aorta’s course. These typically result in retrograde dissections, and the false lumens may thrombose spontaneously if dissections are limited. Catheter-related dissections are most often distal, Type B injuries and can often be managed medically unless the dissection is extensive (Fig. 48.4).
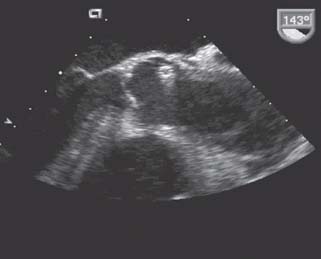
FIGURE 48.4 TEE in a long-axis view demonstrating a case of iatrogenic dissection. A bare metal stent is entrapped in the left main trunk and has caused a retrograde dissection to the aortic sinuses with antegrade propagation distally to the descending thoracic aorta (not shown).
Dissections can also occur during aortic cross clamping, cannulation, or manipulation during cardiac surgery. Such dissections are often proximal, Type A dissections, and are diagnosed and treated urgently and successfully at the time of surgery.
INTRAMURAL HEMATOMA AND PENETRATING AORTIC ULCER
Intramural hematoma and penetrating aortic ulcer are two aortic dissection variants that vary from classic dissection by the absence of a classic intimal flap. Recent advances in diagnostic imaging modalities have led to an increased awareness and better understanding of these entities.
Intramural Hematoma
Intramural hematoma consists of a noncommunicating blood collection in the aortic wall. Unlike a true dissection, there is no loss of intimal continuity, no entry tear, and thus no intimal flap. The pathophysiology may be related to rupture of the aortic vasa vasorum.
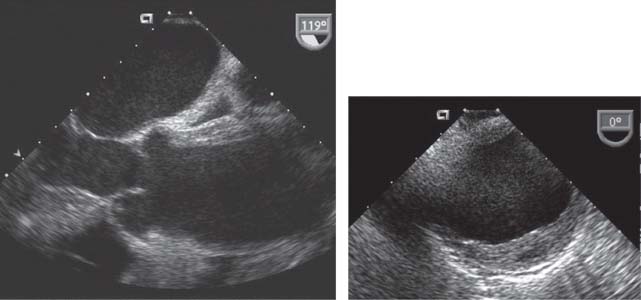
By TEE, intramural hematoma is characterized by absence of a dissection flap, a regional crescent-shaped thickening of the aortic wall usually >0.7 cm, and central displacement of intimal calcium (Fig. 48.5). At times, intramural echolucencies representing noncommunicating pockets of fresh blood can be seen. Distinguishing intramural hematoma from severe atheroma, a thrombosed false lumen, or aneurysm with mural thrombus can be difficult. Angiography is of limited diagnostic accuracy in the evaluation of hematomas, as it fails to image the aortic wall. If the clinical history is concerning, a negative TEE should not represent the final diagnostic evaluation. CT and MRA represent highly accurate imaging modalities that are frequently used as an initial or complementary study in the evaluation of hematomas.
FIGURE 48.5 TEE in both long- and short-axis views showing an intramural hematoma, characterized by no dissection flap, a crescent-shaped thickening of the aortic wall, central displacement of intimal calcium, and echolucent intramural pockets representing intramural blood.