Primary prevention seeks to avoid the occurrence of new infections, i.e., to eliminate the risk of infection of exposed individuals and to interrupt the chain of transmission. Those goals are mainly achieved by means of strategies focusing on the control of both vector- and non-vector-borne transmission. However, in the case of the vertical route, it should be borne in mind that there is no procedure available to reliably impede mother-to-child transmission.
Secondary prevention consists of the screening and detection of individuals infected with T. cruzi in the early stages of disease. This strategy is essential in acute cases. In chronic cases, and more particularly those with the indeterminate form of Chagas disease, particularly asymptomatic individuals who are often unaware of being infected, the aim of secondary prevention is to provide etiological treatment. Eradication or reduction of the parasite load may in principle avoid or delay the progression of disease into the determinate chronic forms and contributes to interrupting the infection’s epidemiological chain of transmission.
Tertiary prevention consists of the clinical interventions performed to limit the morbidity and mortality of established disease in a special manner as concerns CHD.
Primary Prevention of Chagas Disease
Since the era of Carlos Chagas and colleagues, the belief has been held that the control of infection could be more easily attained by means of primary prevention. Currently, primary prevention is still centered on the control of vectors by chemical means, in addition to the screening of blood donors using sensitive and specific serologic methods. Such measures must be continuously supported by strategies targeting basic health education, effective participation of the community in programs, improvement of housing conditions and constant epidemiological surveillance. The reward for such strategies is immediate when they are rigorously implemented, as the number of new vector-borne infections is reduced. The rate of infection through blood transfusions decreases soon afterwards. In areas where vector-borne and blood transfusion transmission are controlled, the number of infected pregnant women is progressively reduced, whereby vertical transmission exhibits a significant tendency towards reduction. The early detection of infection in fetuses allows for specific treatment of the affected children [29, 30]. Moreover, primary prevention strategies targeting care in the laboratory handling of T. cruzi are quite effective by avoiding work accidents. Similarly effective is the adequate screening of organ donors through the application of universal serologic techniques in locations where the seroprevalence is high, or also the use of serologic tests combined with specific epidemiological investigation.
Alternatively, the opportunities to diagnose infection with T. cruzi and to provide etiological treatment before women become pregnant to prevent vertical transmission are rare. Additionally, the prevention of infection by the oral route is practically impossible. Therefore, in both instances, the most effective strategy to control infection consists of early detection and trypanosomicidal treatment.
Preventive immunization by means of a safe and effective vaccine is not yet available, partially as a function of the theoretical risk of adverse immune reactions [31]. Another hindrance is represented by the multiple molecular variants of T. cruzi [32], which confounds the search for an adequate vaccine by a rather indefinite antigenic target [33].
The implementation of control programs depends on three essential factors: acknowledgment of the medical significance of the disease; definition of its social impact; and the availability of resources and minimal strategies aimed at control. Following the most characteristic period of oblivion to Chagas disease, the modern era of combat against the disease began between 1945 and 1955, when residual insecticides were used to combat the vectors and pathologic, serologic and clinical studies became systematized [34]. Beginning in the 1960s, national programs of vector control were launched; serologic screening for T. cruzi in blood banks became mandatory in the 1980s, parallel to the measures established for the control of the emerging HIV pandemic [19, 34].
Control of Vectors
Vector control is essential under conditions of domiciliary and peridomiciliary infestation, by means of continuous and regular application of residual insecticides also followed, in principle, by continuous and sustained epidemiological surveillance. Synthetic pyrethroids derived from chrysanthemic acid (deltamethrin, lambda-cyhalothrin, cyfluthrin, etc.) are currently the most effective insecticides. In cases of resistance to those agents, which seldom occurs, organochlorine or carbamate compounds may be alternatively used [34].
The perception that population-based educational campaigns and active participation of the community in strategies for vector control are an essential part of epidemiological surveillance is universally accepted. Improvement of housing conditions is also quite effective; however, except for rare exceptions (such as the 2001 experience in Venezuela), this has never been properly prioritized in national programs [35].
It should be noted that eradication, namely, complete interruption of Chagas disease transmission, is practically an unattainable epidemiological target. Even in places where that goal could be partially achieved, such as the extinction of transmission by Triatoma infestans in countries such as Uruguay, Chile and Brazil, entomological surveillance must be maintained for many years. The proposal that infection with T. cruzi evolved from a primitive type of zoonosis to a true and highly spread anthropozoonosis is currently very clear. Moreover, the parasite is currently disseminated across many sylvatic areas, in which its ecotopes are being increasingly altered by human activity (e.g., indiscriminate deforestation) such as in the Amazon region, where the incidence of autochthonous cases is increasing [36]. Evidence also exists for the presence of several secondary vectors, namely, triatomine insects potentially susceptible to domiciliation, as well as that of vector resistance to the most commonly used insecticides.
Control of Transmission via Transfusions
Although less rigorous, this strategy was applied as early as the 1990s, through the serologic detection and chemoprophylaxis of suspected blood, and intensified in parallel with the establishment of measures against infection with HIV. Ideally, two highly accurate serologic tests based on two different techniques (e.g., indirect immunofluorescence and ELISA) should be employed for the screening of blood donors. However, starting in 2002, the World Health Organization (WHO) recommends performing only one test (ELISA) to detect blood donors infected with T. cruzi [20]. Likely due to technical inadequacies in the performance of the serologic tests, this recommendation led to a significant reduction in the sensitivity of detecting infected blood and organ donors, the consequences of which are only beginning to be understood. For example, acute Chagas infection was detected in one individual without Chagas disease who received the transplanted liver of an allegedly non-infected donor in Brazil, which is an endemic country [37]. The challenge is heightened by the fact that a large fraction of the donors and recipients are transfused with large amounts of blood components on the occasion of the event leading to death or in the perioperative period of heart transplantation, respectively. Under such circumstances, high-sensitivity serologic control of hemodiluted sera, with sufficient specificity so as not to reject viable organs should be mandatory. The need to improve the methods for serologic detection of T. cruzi infection in endemic and non-endemic areas is reinforced by the recent description of a series of patients with chest pain and segmental wall-motion abnormalities of the left ventricle (such as apical aneurysm) highly suggestive of CHD, but who had negative results by the immunofluorescence serologic test [38].
Prevention of Infection Through Solid Organ Donation
The most common scenario of infection via solid organ donation is the case of a seropositive donor and a seronegative recipient. However, just as in the case cited above [37], the transmission of Chagas infection through organ transplantation (heart, kidneys, bone marrow, liver) was also reported relative to allegedly seronegative donors, even in non-endemic countries.
In the absence of more conclusive and informative evidence, the consensus of specialists converges towards the following prophylactic guidelines based on the serologic status of organ donors and recipients before transplantation [39, 40].
When the donor is seronegative and the recipient is seropositive, the latter’s postoperative recovery should be closely monitored for the early detection of possible infection reactivation, which might occur as a function of the immune modulation regimen required to prevent graft rejection. If parasitemia intensifies or if clinical indicators consistent with reactivation appear, etiological treatment should be initiated. Prophylactic treatment of recipients with trypanosomicidal agents before transplantation is a less favored approach, although it is a reasonable option within the context of heart transplantation [41]. Notably, not even qualitative polymerase chain reaction (PCR) techniques for the detection of parasitemia have sufficient sensitivity to define the presence of reactivation, although their negative predictive value is adequate to rule it out. Quantitative PCR techniques (real–time PCR) are currently used only for research purposes. However, it is safe to assume that once cutoff points are established, this method will be used for the indication of preemptive treatment in cases with high parasitemia levels.
The same principles apply when both donor and recipient are seropositive; as a rule, specific treatment is recommended for the donor before transplant surgery [28]. Relative to heart transplantation, which is not allowed when the donor is seropositive, relative to other organs, prior specific consent is required.
Finally, the situation of a seropositive potential donor and a seronegative recipient (in the case of living donors) is a particular cause of concern because transplantation is a priority that cannot be bypassed. In such cases, it is recommended to administer antitrypanosomal treatment to the donor for at least 10 days (60 days ideally) to reduce or eliminate the parasitemia as well as to the recipient along with treatment for 10 days after surgery to minimize the odds of parasite invasion and multiplication in his/her body. If the recipient becomes seropositive, standard trypanosomicidal treatment for 60–90 days is recommended [28].
Prevention of Laboratory and Hospital Accidents
Training in and compliance with the universally accepted basic principles for work in environments in which professionals work with people or materials that are potentially contaminated with T. cruzi are the core of the prevention of transmission through this route. Ideally, the professionals should be subjected to serologic testing upon being hired by institutions in which they may be exposed to unintentional risk. If contamination is suspected, the following procedures should be adopted: immediate disinfection of the eyes or skin lesions using alcohol; start prophylactic etiological treatment with the usual dose for 10 days; notify the head of the laboratory or hospital unit in which the incident occurred to avoid repetitions; and perform serological testing 30 days after the event. In cases of seroconversion, standard etiological treatment for 60–90 days is recommended [28].
Preventive Treatment for Cases of Congenital Transmission
Preventive etiological treatment using the currently available drugs is contraindicated for seropositive pregnant women. Pregnant women without a previous diagnosis of Chagas disease should be subjected to serological testing. Incases of positive results, the early detection of transmission to and treatment of the infected newborn infants are the basic measures [29, 40]. Newborn infants from mothers with known or highly suspected infection by T. cruzi should be carefully examined for signs of acute Chagas disease. Additionally, parasitemia should be investigated particularly by direct examination of the umbilical cord and the infant’s blood. The conventional serologic tests (based on the detection of IgG antibodies) are not indicated in this stage because the maternal antibodies remain in the infant for approximately 6 months after birth. In contrast, serological tests that investigate IgM antibodies might contribute to the diagnosis because the latter are indicative of active infection.
In confirmed cases of congenital transmission, etiological treatment should be started immediately, which is usually well tolerated by infants.
In the absence of clinical or laboratory evidence of infection, the infants should be subjected to conventional serologic testing (investigating IgG antibodies) at age 6–8 months; only positive cases should be administered etiological treatment. The infants should be then subjected to clinical and laboratory assessments once per year. Persistently negative serologic results are indicative of cure of infection, which usually occurs about one year after the onset of treatment. In contrast, positive results denote therapeutic failure and the infants should be treated again, preferably with a different trypanosomicidal drug [28].
Oral Transmission
Being unpredictable, primary prevention of transmission by the oral route is unfeasible; only progressive improvement of the population’s educational and hygienic levels will allow the control of this mechanism of Chagas disease transmission. Because the parasitic load is usually high, and because the mucosa of the digestive tract is highly permeable to T. cruzi, the mortality in the acute stage of infection may be quite high. Healthcare professionals should develop a high degree of diagnostic suspicion, as atypical cases with gastrointestinal bleeding and myopericarditis with large pericardial effusions are often described, to achieve effective parasitological confirmation and to begin etiological treatment immediately. Similarly, epidemiological surveillance should be established later on, also including the people in contact with patients infected with T. cruzi per the oral route [40].
Secondary Prevention of Chagas Disease
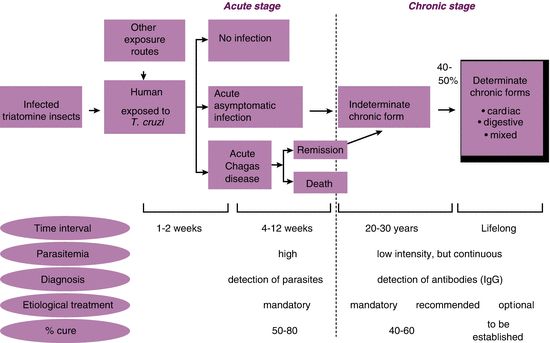
Independent of the route of transmission, human exposure to T. cruzi exhibits three main scenarios. The first, that of no infection, is based on the assumption that a quick and efficient immune response might prevent the settlement of the parasite in the human body. Infection does occur in the other two scenarios, but it may be clinically unapparent (scenario two) or it may manifest the signs and symptoms of the acute stage of Chagas disease (Fig. 2).
Following vector-borne exposure to T. cruzi, the usual incubation period of the acute stage is 1–2 weeks (being variable in other routes of transmission), during which the parasites in trypomastigote (flagellate) form are detectable in the bloodstream by microscopic methods. The acute stage lasts approximately 12 weeks, with most patients being asymptomatic or oligosymptomatic; because the actual cause of infection passes unnoticed, the few (unspecific) symptoms are attributed to other and trivial diseases, such as the flu.
The acute stage of Chagas disease is diagnosed in a very small number of patients, who exhibit signs and symptoms compatible with myocarditis or severe meningoencephalitis. Under such circumstances, the patients are at risk of death (which occurs in 5–10 % of the cases, particularly among small children or when infection is transmitted per the oral route and the parasitic load is intrinsically high).
Once the acute stage ends, the parasitemia becomes practically undetectable by direct examination, even when specific treatment is not administered, as a function of the protective action of the immune system. Then, the chronic stage of CHD begins, with most patients remaining asymptomatic for decades, which characterizes the indeterminate form of the disease. As previously noted, 40–50 % of the patients characteristically develop signs and symptoms of organ involvement 20–30 years after the acute stage of disease, corresponding to the cardiac, digestive or mixed types of CHD [42].
Secondary prevention especially targets the second and third aforementioned scenarios to hinder the progression of the indeterminate form of disease into the clinically determined types. In practical terms, the aim is to avoid the manifestation of CHD, to wit, the most formidable and frequent expression of the disease. The basic strategy to achieve that goal is to provide etiological treatment to the infected individuals, its applicability depending on the active identification of the largest possible number of such patients.
Etiological Treatment
Although specific treatment is the cornerstone of the secondary prevention of Chagas disease, only two compounds admittedly active against T. cruzi are available for clinical use, that is, benznidazole and nifurtimox. Both were developed 40 years ago, and thus, the lack of further investigation of additional drugs bears witness to the neglect of Chagas disease by the medical and scientific community and society at large, in this case represented by governmental agencies and the industrial sector.
Both available drugs have trypanosomicidal activity against the amastigote and more intensively against the circulating trypomastigote forms [43]. Those agents were tested for the treatment of patients in the acute stage of infection, inducing clinical remission and parasitological and serological negative conversion (delayed) in up to 80 % of the cases [44]. Based on such evidence and in the absence of more “definitive” studies, there is essentially universal agreement that etiological treatment should be indicated in all patients diagnosed with acute Chagas disease, independent of the route of transmission [41]. That indication is also unanimously accepted relative to episodes of reactivation in patients in the chronic stage of disease, who usually exhibit natural or iatrogenic immunosuppression.
However, the most propitious scenario of secondary prevention is not represented by the patients diagnosed in the acute stage of disease but by the much larger number of patients with the indeterminate form of chronic disease. That crucial epidemiological fact notwithstanding, the indication for trypanosomicidal treatment in patients in the chronic stage of Chagas disease [45] has been repeatedly discredited and called into question, to the point that consensus is nonexistent in this regard, although it is an essential component of secondary prevention [41]. This position, which is not justified under the current knowledge, is largely due to an erroneous concept, according to which the main pathogenetic mechanism in Chagas disease is autoimmunity, in disregard of the fact that this was challenged long ago [46]. Further arguments for that unfounded position derive from the personal experience of some physicians with the more advanced stages of disease—when, indeed, trypanosomicidal treatment has little to contribute—who refuse to acknowledge the evidence that, although not “definitive”, reasonably supports the fundamental notion that Chagas disease is, in essence, an infectious disease, the etiological agent of which remains in the human body, where it is the direct cause of a low-intensity although practically incessant inflammatory state in some tissues, such as the myocardium.
Multiple and an increasing number of studies indicate that parasite persistence is the essential mechanism that accounts for the establishment of inflammatory tissue lesions either directly or mediated by the immune system. Such lesions damage the contractile myocardium and the specialized system generating and conducting cardiac electrical activity, causing cell necrosis and intensive reactive and reparative fibrosis [4, 47]. Thus, it is natural to speculate that antitrypanosomal treatment in the non-advanced chronic stage of CHD may favorably modify the natural history of the disease [48]. The underlying hypothesis is that elimination, or at least reduction, of the parasitic load may attenuate and/or delay the progression of myocarditis in the chronic stage of CHD. That basic notion reflects the theory that autoimmune aggression (disregarding the presence of parasites in the tissues) is not the decisive mechanism in the pathogenesis of CHD [49].
Although still a controversial subject, etiological treatment should be administered, as a rule, to most patients with the indeterminate and the cardiac and digestive forms of disease in the non-advanced stages [48, 49]. In some South American countries, this indication became official public health policy. This recommendation is also considered fundamental in the United States, based on studies, seminars and a systematic literature review conducted by investigators at the Centers for Disease Control and Prevention (CDC) and Latin American collaborators [50]. This position is supported by a systematic review with meta-analysis of the few randomized studies performed with asymptomatic infected patients—presumably most of them with the indeterminate form of disease. The results indicated that etiological treatment (particularly with benznidazole) is beneficial in as much as it improves the host-parasite relationship, resulting in negative conversion of the xenodiagnosis and the reduction of circulating anti-T. cruzi antibodies, as demonstrated by the serologic tests [51, 52]. The two most conclusive studies included in that meta-analysis were performed in children; the results demonstrated seroconversion and cure of infection in approximately 60 % of the participants after follow-up of 3–4 years [53, 54]. Children characteristically tolerate trypanosomicidal treatment better than adults.
Relative to infected individuals with clinical and laboratory abnormalities demonstrating established CHD, many investigators consider that specific treatment should still be offered, except in cases of highly advanced myocardial injury. That position is based on several lines of evidence that taken together tip the balance in favor of this strategy:
evidence resulting from experimental models of infection with T. cruzi collected by different groups of investigators, according to which etiological treatment attenuates the progression of heart disease, although complete eradication of the parasite was not attained (the parasitic load was merely reduced) [55–57];
the side effects of either available trypanosomicidal agent occur less frequently and are better tolerated than was previously believed; moreover, such undesirable effects—gastrointestinal and skin reactions, polyneuropathy, leukopenia—might be considered as tolerable and reversible, thus contrasting with the beneficial potential of short-term treatment (2–3 months) [58–59];
the results of several observational studies that assessed the effect of etiological treatment in patients with heart disease and applied clinically relevant outcome measures point to a true positive effect, with favorable modification of the natural history of the disease [60–64];
a meta-analysis that included three randomized and six observational studies concluded that the patients treated with benznidazole exhibited significant risk reduction of presenting clinical events over time compared to the patients not subjected to etiological treatment (odds ratio: 0.29; 95 % confidence interval: 0.16–0.53) [65].
To summarize, based on the actions and individual positions of many investigators [58, 66, 67], and on official statements by agencies responsible for developing health policies, an emergent current convergence favors the position that etiological treatment should be made available to most infected patients in the chronic stage of Chagas disease [25, 40, 50, 68]. This perspective is based on the concept that as a function of the currently available knowledge and while still awaiting the conclusive evidence of the BENEFIT randomized study [69], the risk of incurring an alpha error (not to apply a promising therapeutic intervention with tolerable side effects) is much less acceptable than incurring a beta error (not to adopt something that might prove futile in the future) [41]. In fact, even to basic researchers, the indifference of doctors that precludes them from even considering the possibility of indicating etiological treatment for their patients is questionable from an ethical standpoint [70].
The implementation of effective secondary prevention measures at the population level must include active screening of children of infected mothers, relatives and other individuals exposed to infection in endemic areas. Additionally, the diagnostic opportunities provided by screening in blood banks, organ donations and job hiring processes should be maximized.
New trypanosomicidal drugs may possibly become available for clinical use in the near future, whether prescribed alone or in combination with the two agents known to be effective. Some of the drugs found promising in preclinical trials include posaconazole [71], ravuconazole [72] and fexinidazole [57]. However, itraconazole and allopurinol are not promising, due to the lack of sufficient favorable evidence or clearly negative results [73].
Finally, one should hope that the supply of benznidazole and nifurtimox does not become jeopardized by management problems among the industrial suppliers [74].
Tertiary Prevention of Chagas Disease
The fundamental issue in the tertiary prevention of Chagas disease is to reduce its inherent mortality, which is the outcome exhibited by 40–50 % of the individuals infected with T. cruzi. Those are the patients who, in the chronic stage, progress to the determined cardiac, digestive and mixed forms of the disease [48, 49]. The digestive form manifests as megaesophagus and/or megacolon in 15–20 % of the cases, independent of an association with cardiovascular involvement. There are no data on the mortality, specifically attributable to the involvement of the digestive tract, with no fully effective clinical or surgical treatment for symptoms such as dysphagia, odynophagia, constipation and malnutrition.
The cardiac form is the most severe and frequent clinical manifestation of Chagas disease by far, affecting 30–40 % of the infected individuals during the many decades of progression of the chronic disease stage. It is the most prevalent cardiomyopathy in Latin America and the first cause of cardiovascular mortality among individuals aged 30–50 years in endemic areas [42].
The manifestations of cardiovascular involvement in the chronic stage of Chagas disease correspond to three clinical syndromes that often coexist in one and the same patient: ventricular dysfunction progressing to heart failure, rhythm disorders, and systemic and pulmonary thromboembolic complications. Whereas all three syndromes contribute to the high mortality associated with Chagas disease, sudden death, which has particular clinical relevance, is significantly related to arrhythmias that cause bradycardia and/or tachycardia, which frequently coexist in one and the same individual.
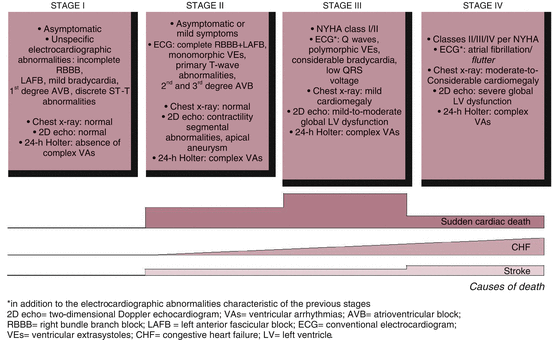
The progression of CHD might schematically be described as four sequential stages (Fig. 3).
In stage one, the patients are asymptomatic, as they were during the indeterminate form, whereas the presence of cardiomyopathy is usually revealed by a few relevant electrocardiographic (ECG) abnormalities: sinus bradycardia, first-degree atrioventricular block, incomplete right bundle branch block and nonspecific ventricular repolarization abnormalities. In a small proportion of cases, the ECG is completely normal, whereas diagnostic imaging tests disclose discrete segmental wall-motion abnormalities of the left and/or right ventricle, with the left ventricular global systolic function being preserved [75]. Other possible functional changes include ventricular diastolic dysfunction—most likely caused by sparse areas of fibrosis—on the echocardiogram [76] and parasympathetic denervation on Holter monitoring or other autonomic tests [77]. In many cases, during this stage, the patients are characteristically able to undergo intense physical exertion, manifesting vague symptoms only.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree