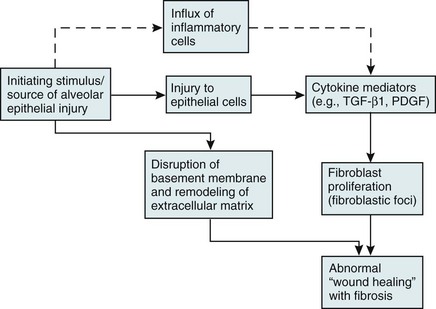
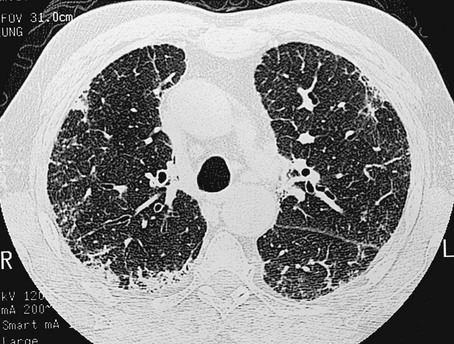
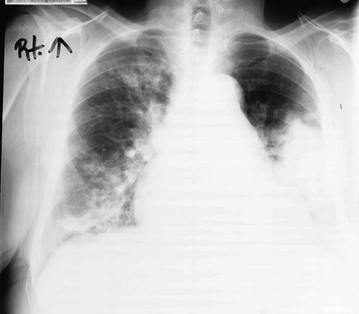
11 Approximately 65% of patients with diffuse parenchymal lung disease are victims of a process for which no etiologic agent has been identified, even though a specific name may be attached to the disease entity. Included in this category are idiopathic pulmonary fibrosis, pulmonary fibrosis associated with connective tissue disease, sarcoidosis, pulmonary Langerhans cell histiocytosis, and a variety of other disorders. Many general aspects of these problems were discussed in Chapter 9. This chapter focuses on the specific diseases and their particular characteristics. Over the past several years, a newer conceptual framework has emerged. According to the newer theory, alveolar inflammation does not play a critical role in the eventual development of fibrosis. Rather, fibrosis is believed to result directly from alveolar epithelial injury and is thought to be a manifestation of abnormal wound healing within the lung parenchyma. According to the newer paradigm, injury to alveolar epithelial cells (still from an unidentified source or agent) is the primary initiating event. Whereas injury to type I alveolar epithelial cells normally would be followed by a repair process that includes proliferation of type II cells and differentiation into type I cells, this repair process is impaired, at least in part because of disruption of the basement membrane, which normally is important for the reepithelialization process. At the same time, alveolar epithelial cells express a variety of profibrotic cytokines and growth factors, including platelet-derived growth factor (PDGF) and transforming growth factor (TGF)-β1, which enhance fibroblast migration and proliferation. Fibroblastic foci develop at sites of alveolar injury and appear to be responsible for increased extracellular matrix deposition. This process is summarized in Figure 11-1. The chest radiograph shows an interstitial (reticular) pattern that is generally bilateral and relatively diffuse but typically is more prominent at the lung bases, particularly in the peripheral subpleural regions (see Fig. 3-6). Neither pleural effusions nor hilar enlargement is common on the radiograph. High-resolution computed tomography (HRCT) scanning often has a characteristic appearance, showing interstitial densities that are patchy, peripheral, subpleural, and associated with small cystic spaces (Fig. 11-2). The pattern of small cystic peripheral abnormalities on HRCT is termed honeycombing and indicates irreversible fibrosis. Many patients have serologic abnormalities, such as a positive test result for antinuclear antibodies, which are generally found in patients with autoimmune or connective tissue disease. However, in the absence of other suggestive clinical features, these abnormalities are thought to be nonspecific and not indicative of an underlying rheumatologic disease. The diagnosis is definitively made by surgical lung biopsy, but only in the appropriate clinical setting when other etiologic factors for interstitial lung disease cannot be identified. Some patients are too frail for lung biopsy, and if HRCT scan shows the classic pattern of honeycombing, the diagnosis can be made with relative certainty without a lung biopsy. The histologic expression of IPF is in the form of usual interstitial pneumonia (UIP) (see Fig. 9-3), and patients who have a pathologic pattern more compatible with desquamative interstitial pneumonia or nonspecific interstitial pneumonia (see Other Idiopathic Interstitial Pneumonias; also see Chapter 9) should not be considered to have IPF. Granulomas should not be seen on an IPF biopsy specimen. If they are found, granulomas indicate the presence of another disorder. Several other disorders besides IPF fall under the category of the idiopathic interstitial pneumonias and have often been confused with IPF. Although these disorders are uncommon, some are briefly described here, largely to clarify how their pathologic features differ from UIP and how their clinical features differ from IPF. They are also mentioned in Chapter 9 as part of the discussion on the pathology of the interstitial pneumonias. Cryptogenic organizing pneumonia is a disorder characterized by connective tissue plugs in small airways accompanied by mononuclear cell infiltration of the surrounding pulmonary parenchyma. As noted in Chapter 9, the terms cryptogenic organizing pneumonia (COP) and bronchiolitis obliterans with organizing pneumonia (BOOP) have often been used interchangeably, but the term BOOP is best reserved for the pathologic picture rather than the clinical syndrome. Although the histologic picture of BOOP can be associated with connective tissue disease, toxic fume inhalation, or infection, the large majority of cases have no identifiable cause and are considered idiopathic. The term COP is most appropriate for patients who have “idiopathic BOOP”—that is, the histologic pattern of BOOP but no apparent cause for this pattern. Like chronic eosinophilic pneumonia (see later), COP often has a subacute presentation (over weeks to months) with systemic (constitutional) as well as respiratory symptoms. The chest radiograph shows patchy infiltrates, generally with an alveolar rather than an interstitial pattern, often mimicking a community-acquired pneumonia (Fig. 11-3). Like chronic eosinophilic pneumonia, the response to corticosteroids is often dramatic and occurs over days to weeks. Therapy is usually prolonged for months to prevent relapse. Acute interstitial pneumonia (AIP) is a more acute or fulminant type of pulmonary parenchymal disease that begins with the clinical picture of acute respiratory distress syndrome (ARDS; see Chapter 28) but without any of the usual inciting events associated with development of ARDS. Imaging studies of AIP typically show features of ARDS, including areas of ground-glass opacification and alveolar filling (as opposed to a purely interstitial pattern). The histologic pattern is that of diffuse alveolar damage, often showing some organization and fibrosis. Although mortality is high overall, a small percentage of patients do well, with clinical resolution of the disease and no long-term sequelae. Progressive systemic sclerosis, or scleroderma, is a disease with the most obvious manifestations located in the skin and small blood vessels. Other organ systems, including the gastrointestinal tract, lungs, kidneys, and heart, are involved relatively frequently. Of all the connective tissue diseases, scleroderma is the one in which pulmonary involvement tends to be most severe and most likely associated with significant scarring of the pulmonary parenchyma. Pulmonary fibrosis complicating scleroderma appears to be strongly associated with the presence of a particular serologic marker, an autoantibody to topoisomerase I (antitopoisomerase I, also called Scl70). Another potential pulmonary manifestation of scleroderma is disease of the small pulmonary blood vessels, producing pulmonary arterial hypertension, which is discussed in Chapter 14. This involvement appears to be independent of the fibrotic process affecting the alveolar walls.
Diffuse Parenchymal Lung Diseases of Unknown Etiology
Idiopathic Pulmonary Fibrosis
Other Idiopathic Interstitial Pneumonias
Pulmonary Parenchymal Involvement Complicating Connective Tissue Disease
Thoracic Key
Fastest Thoracic Insight Engine