The introduction of optical coherence tomography has provided a new method for evaluating the vascular response to drug-eluting stents (DESs). We used optical coherence tomography to compare neointimal coverage and stent malapposition among DESs in patients with ST-segment elevation myocardial infarction. Optical coherence tomography was performed at 9 months after implantation of 3 types of DESs at the culprit lesions in 46 patients with ST-segment elevation myocardial infarction (16 sirolimus-eluting stents [SESs, Cypher Select], 11 paclitaxel-eluting stents [PESs, Taxus Liberte], and 19 zotarolimus-eluting stents [ZESs, Endeavor Sprint]). The neointimal thickness and apposition at each strut at each 1-mm interval and the presence of thrombi in each stent were evaluated. A total of 11,512 stent struts were analyzed. SESs had the thinnest neointimal thickness (SES 62 ± 43 μm vs PES 244 ± 142 μm vs ZES 271 ± 128 μm, p <0.001). The incidence of uncovered struts and malapposed struts were significantly greater in SESs and PESs than in ZESs (SES vs PES vs ZES, 16.2 ± 17.8% vs 4.7 ± 7.4% vs 0.6 ± 1.5%, respectively, p = 0.001; and 4.0 ± 8.2% vs 2.1 ± 4.5% vs 0 ± 0%, respectively, p = 0.001). Thrombus was also detected more often in SESs and PESs than in ZESs (SES, 6 [38%] vs PES, 3 [27%] vs ZES, 1 [5%], p = 0.02). In conclusion, the rate of stent strut coverage and malapposition were significantly different among the DES types in ST-segment elevation myocardial infarction. In particular, most stent struts in ZESs were covered with neointima and well-apposed. These findings imply that the type of DES might affect the vascular response in thrombotic lesions of ST-segment elevation myocardial infarction.
A recent autopsy study has shown that vessel healing after drug-eluting stent (DES) implantation at the culprit site in patients with acute myocardial infarction is substantially delayed compared to that in patients with stable angina. However, little data have been reported from intravascular imaging modalities with regard to arterial healing among different types of DESs in ST-segment elevation myocardial infarction. The introduction of intravascular optical coherence tomography (OCT) has provided new opportunities for the evaluation of stents in vivo at a micron-scale level. We compared the neointimal coverage and stent malapposition among 3 types of DESs in patients using OCT at 9 months after implantation.
Methods
From January 2007 to March 2008, 120 patients with ST-segment elevation myocardial infarction were treated by percutaneous coronary intervention using DES implantation at Severance Hospital, Yonsei University (Seoul, Korea). Of these 120 patients, 46 (39 men and 7 women, mean age 58 years) who met the inclusion and exclusion criteria were followed up by OCT at 9 ± 2 months after implantation. The inclusion criteria for the present study were a de novo culprit lesion treated with primary percutaneous coronary intervention with a sirolimus-eluting stent (SES, Cypher Select, Cordis, Miami Lakes, Florida), paclitaxel-eluting stent (PES, Taxus Liberte, Boston Scientific, Natick, Massachusetts), or zotarolimus-eluting stent (ZES, Endeavor Sprint, Medtronic, Santa Rosa, California) in ST-segment elevation myocardial infarction and a native vessel size 2.5 to 3.5 mm in diameter. The exclusion criteria were significant left main coronary artery disease, in-stent restenosis (defined as >50% diameter stenosis at follow-up angiography), congestive heart failure or low ejection fraction (≤35%), renal insufficiency with baseline creatinine ≥2.0 mg/dl, and lesions unsuitable for OCT (vessel size ≥4.0 mm or proximal lesion location 15 mm from the ostium of each artery). Of the 120 patients, OCT at 9 (± 2 months) could be performed in only 50 patients. OCT could not be performed in 70 patients; in 57 patients because of technical difficulties (34 with a proximal lesion <15 mm from the ostium, 23 with a large vessel size >4.0 mm), 8 patients had a low ejection fraction of ≤35%, and 5 patients refused to undergo OCT. Additionally, 4 stents were not completely evaluated for the whole stent length. The institutional ethics committee of Severance Hospital approved the study protocol, and all patients had given written consent before the procedure. All patients took 250 mg aspirin and 600 mg clopidogrel before primary percutaneous coronary intervention and 100 mg aspirin and 75 mg clopidogrel thereafter for ≥9 months.
Quantitative coronary angiography analysis was performed using an off-line quantitative coronary angiographic system (CASS System, Pie Medical Instruments, Maastricht, The Netherlands) by a single person who was unaware of the patient information and stent type. The minimal luminal diameter of the treated coronary segments, reference vessel diameter, percentage of stenosis diameter, and lesion length were measured in the view that was the most severe and not foreshortened. The post-stent and follow-up angiograms were evaluated in a similar manner.
A time-domain optical coherence tomographic system (Model M2 Cardiology Imaging System, LightLab Imaging, Westford, Massachusetts) and 0.014-inch wire-tip imaging catheter (ImageWire, LightLab Imaging, Westford, Massachusetts) were used in the present study. A 6Fr or 7Fr guiding catheter was introduced into the coronary artery using a femoral or radial approach. During image acquisition, the occlusion balloon (Helios, Avantec Vascular, Sunnyvale, California) was inflated to 0.4 to 0.6 atm, and Ringer’s lactate was infused at 0.5 to 1.0 ml/s. The image wire was automatically pulled back from distally to proximally, and continuous images were stored digitally for subsequent analysis.
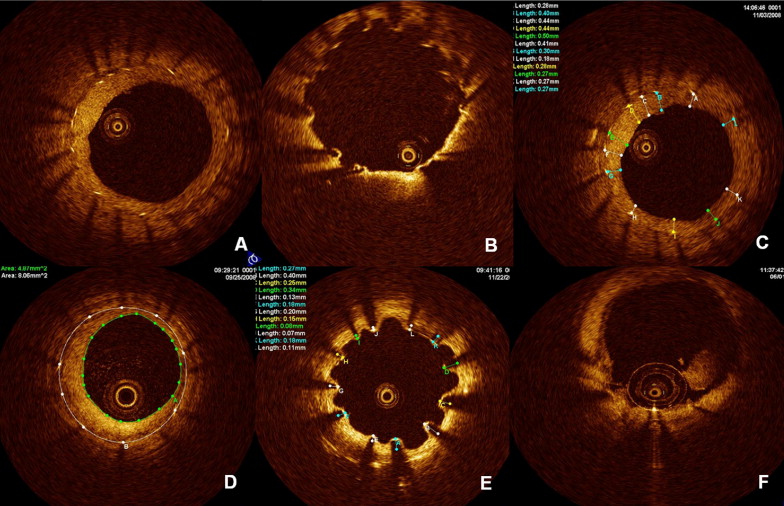
Off-line analysis of the continuous cross-sections within the stented segment was performed at 1-mm intervals (every 15 frames). The strut apposition and neointimal coverage were assessed for each stent strut. The stent area and lumen area were measured, and the percentage of neointima area was calculated as follows: [(stent area − lumen area)/stent area] × 100 ( Figure 1 ). Distances (neointima thickness) between the endoluminal surface of the neointima and stent strut were measured as perpendicularly as possible to the neointima and strut. When no definite neointima was found over the stent strut (<10 μm owing to optical coherence tomographic resolution), it was defined as an uncovered strut. Distances between the strut reflection (total strut thickness + 1/2 of the blooming for stent blooming) and vessel wall were measured as perpendicularly as possible to the strut and vessel wall. The position of the stent strut on the vessel wall was measured by magnifying the individual strut to maximize accuracy when the strut was not fully attached to the vessel wall as determined by visual estimation. Stent malappositions were defined as struts with detachment from the vessel wall ≥160 μm in SESs, ≥130 μm in PESs, and ≥110 μm in ZESs. Thrombus was defined as signal-rich, low-backscattering protrusions or high-backscattering protrusions inside the lumen of the artery with signal-free shadowing on the optical coherence tomographic image. To distinguish thrombi from neointimal dissection or plaque protrusion, we included any protruding mass >250 μm in maximum length and with surface irregularity ( Figure 1 ). Stent overlapping segments and bifurcation lesions with major side branches (≥2.0 mm in diameter) were excluded from the present analysis.
The categorical variables are presented as the mean ± SD or number (%). Analysis of variance was performed to compare continuous variables. If the distributions were skewed, a nonparametric test was used for continuous variables. The categorical variables were compared using the chi-square test or Fisher’s exact test when the expected frequency was <5. Statistical evaluation was performed using Statistical Analysis Software, version 9.1.3 (SAS Institute, Cary, North Carolina). A p value <0.05 was considered statistically significant.
Results
Table 1 lists the baseline characteristics of the study subjects. The 46 stents at the culprit lesions in 46 patients with ST-segment elevation myocardial infarction were evaluated using OCT at 9 months after DES implantation (16 SESs, 11 PESs, and 19 ZESs). SESs were more commonly implanted in the left anterior descending artery, and right coronary artery lesions were more ZESs implanted. No other significant differences were found in the baseline variables among the 3 DES groups.
| Variable | SES (n = 16) | PES (n = 11) | ZES (n = 19) | p Value |
|---|---|---|---|---|
| Age (years) | 57 ± 9 | 56 ± 11 | 59 ± 10 | 0.61 |
| Men | 14 (88%) | 10 (91%) | 15 (79%) | 0.47 |
| Hypertension ⁎ | 6 (38%) | 3 (27%) | 9 (47%) | 0.53 |
| Diabetes mellitus | 6 (38%) | 1 (9%) | 8 (42%) | 0.72 |
| Smoking history | 7 (44%) | 6 (55%) | 5 (26%) | 0.32 |
| Hypercholesterolemia † | 6 (38%) | 4 (36%) | 9 (47%) | 0.55 |
| B2/C lesion type | 16 (100%) | 11 (100%) | 18 (95%) | 0.48 |
| Coronary artery treated | ||||
| Left anterior descending artery | 15 (94%) | 6 (55%) | 9 (47%) | 0.01 |
| Left circumflex artery | 0 (0%) | 2 (18%) | 1 (5%) | 0.59 |
| Right coronary artery | 1 (6%) | 3 (27%) | 9 (47%) | 0.01 |
| Initial TIMI grade 0-1 flow | 11 (69%) | 7 (64%) | 10 (53%) | 0.61 |
| Intracoronary thrombus | 14 (88%) | 10 (91%) | 15 (79%) | 0.63 |
⁎ Defined as history of hypertension and treated with medication or blood pressure >140 mm Hg systolic or >90 mm Hg diastolic on ≥2 occasions or current use of antihypertensive medication.
† Defined as history of hypercholesterolemia or total cholesterol >200 mg/dl or low-density lipoprotein ≥130 mg/dl or high-density lipoprotein <40 mg/dl.
As listed in Table 2 , relatively small-size vessels were more often implanted with SESs than with PESs or ZESs. However, no significant differences were found in minimal luminal diameter when the quantitative coronary angiographic data were compared among the 3 stent groups at follow-up angiography.
| Variable | SES (n = 16) | PES (n = 11) | ZES (n = 19) | p Value |
|---|---|---|---|---|
| Balloon/artery ratio | 1.14:1 | 1.13:1 | 1.13:1 | 0.92 |
| Mean stent diameter (mm) | 2.8 ± 0.2 | 3.1 ± 0.3 ⁎ | 3.1 ± 0.4 ⁎ | 0.02 |
| Mean stent length (mm) | 24.7 ± 5.9 | 29.1 ± 5.9 | 25.0 ± 5.1 | 0.16 |
| Final TIMI grade 3 | 13 (81%) | 10 (91%) | 18 (95%) | 0.43 |
| Quantitative coronary angiographic data | ||||
| Before intervention | ||||
| Mean RVD (mm) | 2.7 ± 0.4 | 2.8 ± 0.4 | 2.7 ± 0.4 | 0.87 |
| MLD (mm) | 0.3 ± 0.5 | 0.5 ± 0.5 | 0.4 ± 0.5 | 0.60 |
| After intervention | ||||
| Mean RVD (mm) | 2.8 ± 0.3 | 2.9 ± 0.3 | 2.8 ± 0.4 | 0.92 |
| MLD (mm) | 2.4 ± 0.7 | 2.8 ± 0.3 ⁎ | 2.8 ± 0.4 ⁎ | 0.03 |
| Acute gain (mm) | 2.3 ± 0.6 | 2.4 ± 0.6 | 2.4 ± 0.6 | 0.75 |
| Follow-up | ||||
| Mean RVD (mm) | 2.7 ± 0.4 | 2.7 ± 0.5 | 2.7 ± 0.4 | 0.98 |
| MLD (mm) | 2.3 ± 0.3 | 2.3 ± 0.5 | 2.1 ± 0.5 | 0.34 |
| Late loss (mm) | 0.2 ± 0.3 | 0.5 ± 0.5 | 0.7 ± 0.4 ⁎ | 0.002 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


