Fig. 6.1
Tuberculosis. Transbronchial lung biopsy from an elderly individual with cough and weight loss. Cultures grew M. tuberculosis. (a) This ill-defined histiocytic infiltrate is vaguely granulomatous. Compare with Fig. 6.2a. (b) Ziehl–Neelsen stain shows several acid-fast bacteria
Detection of AFB is a key responsibility of the pathologist. AFB are tiny rod-shaped structures that stain red (in a blue background) on acid-fast stains such as Ziehl–Neelsen or Fite (Fig. 6.1a, b). As stated above, M. tuberculosis and non-tubercular mycobacteria cannot be differentiated histologically. Although some cases may show large numbers of organisms, AFB are often very few and difficult to find in immunocompetent individuals. Screening with a 20× objective (or lower) may miss organisms if they are few in number. Examination with a 40× or 100× (oil immersion) objective lens for several minutes is required in most cases. Mycobacteria are most commonly found within the necrotic center of necrotizing granulomas, although (unlike Histoplasma) they can also be found in the granulomatous rim. Each necrotic area in the slide should be examined meticulously with constant adjustment of fine focus so as not to miss organisms that may be visible in only one plane. Areas other than the necrotic center and the granulomatous rim virtually never contain organisms.
Non-tubercular Mycobacterial Infection
Clinical and Radiologic Findings
Tuberculosis is such a well-recognized cause of pulmonary granulomas that few pathologists think of non-tubercular mycobacteria (mycobacteria other than M. tuberculosis) as a cause of granulomatous inflammation in the lungs. Clinicians and pathologists are most familiar with these organisms in the setting of acquired immunodeficiency syndrome (AIDS), in which low CD4 counts predispose to uncontrolled proliferation of these organisms within macrophages. It is not widely recognized that infections with these organisms frequently involve immunocompetent patients [12]. In fact, in the United States, non-tubercular mycobacteria are now more commonly isolated than M. tuberculosis, a trend that is reflected in lung specimens seen by pathologists [3, 12]. For unclear reasons, there is a curious predilection for the right middle lobe and lingula of middle-aged women (“middle lobe syndrome” or “Lady Windermere syndrome”) [13, 14]. However, these infections can occur at any age and involve both genders. The most typical radiographic picture is multifocal bronchiectasis with multiple small nodules, but cavities and infiltrates are also common. The diagnosis is most often made by identifying non-tubercular mycobacteria in microbiologic cultures of respiratory tract specimens. The most common organism is Mycobacterium avium–intracellulare (MAI), currently known as MAC. Other species such as M. kansasii, M. abscessus, M. fortuitum, and M. chelonae are occasionally isolated. Since non-tubercular mycobacteria are common in the environment (especially in water), a common clinical dilemma is whether the organisms are truly responsible for the patient’s symptoms or are merely contaminants. There is no easy answer to this question, but clinical criteria to differentiate true infections from saprophytic growth have been proposed [12]. As with tuberculosis, the role of surgical pathologists is to exclude malignancy, identify granulomas, and find mycobacteria on acid-fast stains.
Histologic Findings in Small Biopsies
The histologic findings of non-tubercular mycobacterial infections are highly variable. They include necrotizing granulomas identical to tuberculosis, non-necrotizing granulomatous inflammation associated with organizing pneumonia (“organizing granulomatous pneumonia”), and other nonspecific findings such as bronchiectasis and chronic bronchiolitis [10, 15, 16]. Sheets of foamy macrophages or ill-defined granulomatous infiltrates—rather than well-formed granulomas—are characteristic of infections in immunocompromised hosts, most typically patients with AIDS. The macrophages in such patients are usually packed with large numbers of AFB (Fig. 6.2a, b). The finding of granulomas within airspaces (alveolar spaces and the lumens of small bronchioles) is a clue to non-tubercular mycobacterial infection (see “Hot Tub Lung” section) [15]. AFB may be found in some cases in immunocompetent individuals, but many are negative because of limited sampling or small numbers of organisms. In such cases, cultures may yield organisms even in the face of negative histologic special stains [3, 10]. Therefore, clinicians and pathologists must ensure that results of cultures are negative before an active mycobacterial infection is excluded—this can take up to several weeks. Cultures are the only definitive means for differentiating tuberculosis from non-tubercular mycobacterial infection. For a discussion on examination of acid-fast stains and mycobacterial speciation, see the preceding section on tuberculosis.
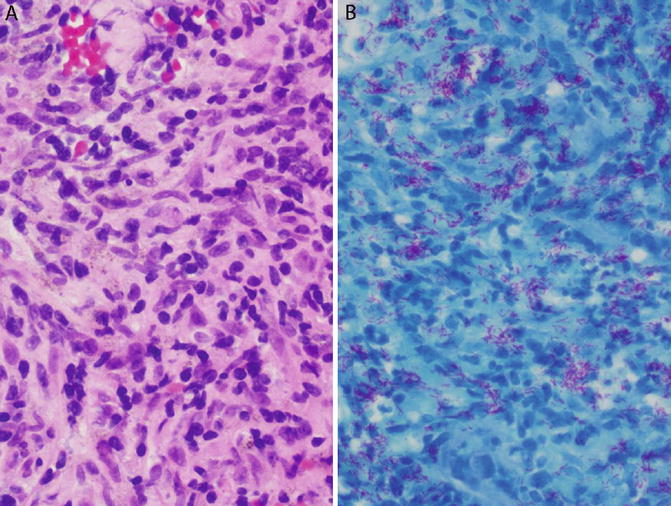

Fig. 6.2
Non-tubercular mycobacterial infection in an immunocompromised patient. Transbronchial lung biopsy. Cultures grew M. avium. (a) Histiocytic infiltrate, vaguely granulomatous. Such lesions often contain greater numbers of organisms than well-formed granulomas. (b) Innumerable acid-fast bacteria on a Ziehl–Neelsen stain
Histoplasmosis
Clinical and Radiologic Findings
Histoplasmosis is the most common endemic fungal infection in the United States. It occurs in several central, midwestern, and southern states along the Ohio and Mississippi river valleys, and also in regions on both sides of the St. Lawrence Seaway, which runs between Canada and the northeastern United States. The infection is also endemic in several countries in Central and South America.
The most common circumstance in which pathologists encounter Histoplasma is a biopsy of a lung nodule to rule out malignancy (Fig. 6.3a, b). The majority of such nodules are incidentally detected on radiographs performed for unrelated reasons. The term “histoplasmoma” has been applied to such nodules when they are shown histologically to be necrotizing granulomas containing Histoplasma organisms [6, 9, 17, 18]. Several studies have demonstrated that the organisms in the majority of these lesions are nonviable and culture negative [3, 7, 10, 19]. The suffix “-oma” is applied because these lesions can mimic many features of malignant lung nodules. Clinicians and radiologists are trained to recognize small, stable (nongrowing), calcified, non-spiculated nodules in nonsmokers as benign [20], but lesions that stray from this norm may be considered suspicious for malignancy. Thus, a solitary lung nodule in a smoker or a nodule that is spiculated, noncalcified, enlarging in size, or positron emission tomography (PET) positive is liable to be biopsied. Since each of these features has been documented in pulmonary histoplasmomas, histology is the only modality that can definitively determine the nature of such nodules. This type of nodule is usually peripheral and is best sampled with a needle biopsy [2, 18, 21]. Surgical pathologists see histoplasmomas in institutions where core needle biopsies of lung nodules are performed, whereas in centers where fine needle aspiration is the preferred approach to peripheral lung nodules, this lesion falls into the domain of the cytopathologist.
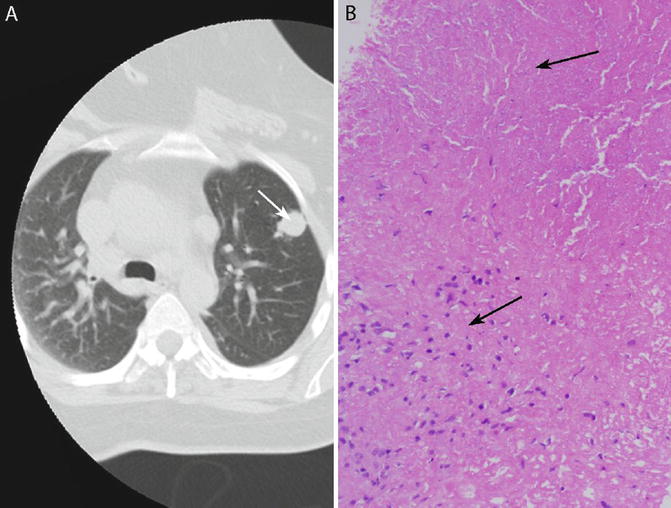

Fig. 6.3
Histoplasmoma. (a) Chest CT showing solitary lung nodule (arrow). (b) Core needle biopsy of the nodule shows a necrotizing granuloma. Necrosis is at the top (arrow) and granulomatous inflammation is at the bottom left (arrow). No organisms are visible on H&E
Rarely, transbronchial or needle biopsy of the lung may be performed in patients with a potentially fatal, progressive form of histoplasmosis known as disseminated histoplasmosis [5]. Such patients are usually immunocompromised and develop disseminated disease that may secondarily involve the lungs, analogous to miliary tuberculosis. In a few cases, clinical and radiologic evidence of extrapulmonary involvement is absent at presentation, making the diagnosis of disseminated disease challenging. Clinical clues suggestive of disseminated disease include the immunosuppressed state of the patient combined with the presence of fever, thrombocytopenia, elevated transaminases, multiple lung nodules, and/or bilateral reticulonodular lung infiltrates [5].
Histologic Findings in Small Biopsies
Histologically, a histoplasmoma is a necrotizing granuloma, the tissue reaction being indistinguishable from the so-called caseating granuloma of tuberculosis (Fig. 6.3a, b) [6, 9]. The lesion is characterized by a large necrotic center surrounded by a relatively thin granulomatous rim composed of a few layers of epithelioid histiocytes, occasionally accompanied by a few multinucleated giant cells. Since the majority of the lesion consists of necrosis, the latter is often the most prominent finding in needle biopsies [2, 18, 21]. The necrosis may be pink (“caseous”) or may contain nuclear debris (“dirty”) or ghosts of necrotic alveoli (“infarct like”). Histoplasma yeasts are present within the necrosis, but are almost never visible on H&E, a diagnostically valuable feature that differentiates Histoplasma from other fungal yeasts that cause pulmonary granulomas, all of which are visible on H&E [1, 6, 7, 9]. GMS staining is required to demonstrate Histoplasma organisms within the necrosis, where they appear as small (3–5 μm), uniform, oval, round, or tapered yeasts (Fig. 6.4a, b) [1, 18]. Organisms can vary from numerous to rare [5]. The size, shape, and uniformity of the yeasts, as well as their non-visibility within necrosis on H&E, are the key diagnostic features, aiding to distinguish them from Cryptococcus and Pneumocystis, which are the fungi most likely to cause diagnostic difficulties [1, 22]. Narrow-based budding is a helpful feature, but is also seen in Cryptococcus, and may not be present when organisms are few in number. Potential explanations for the non-visibility of Histoplasma in histoplasmomas on H&E include the relatively few organisms, necrotic background, extracellular location, and non-viability of the organisms [5].
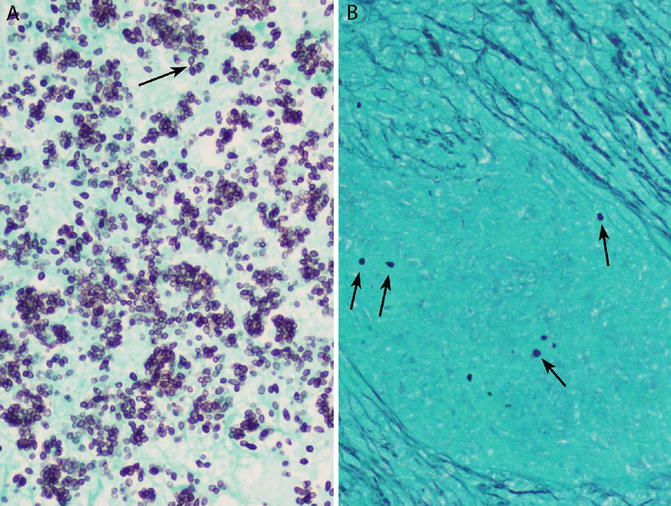

Fig. 6.4
Histoplasma yeasts on GMS. (a) When organisms are numerous, narrow-based budding is likely to be present (arrow). (b) Scarce organisms (arrows) may be missed, especially at low magnification
Histoplasma must also be differentiated from tiny, lightly basophilic microcalcifications, which are often visible within necrosis on H&E, but are nonuniform in shape and GMS negative. Dust particles, which are GMS positive, can mimic Histoplasma, but they are smaller and less uniform and lack the typical oval or tapered shapes of this organism. Conversely, if GMS-stained slides are examined only at low magnification, Histoplasma yeasts can be misinterpreted as dust particles.
The histologic features of the disseminated form of histoplasmosis, which can involve the lung, are highly suggestive of aggressive, widespread disease and serve as a histologic surrogate marker of immunosuppression. They include filling of airspaces and alveolar septa by numerous histiocytes engorged by large numbers of yeasts that are easily visible on H&E (Fig. 6.5a, b) [5, 23]. Granulomas are usually absent, although necrosis may be present. The visibility of the organisms within viable macrophages on H&E is in sharp contrast to the morphology of the far more common histoplasmomas described in the preceding paragraphs.
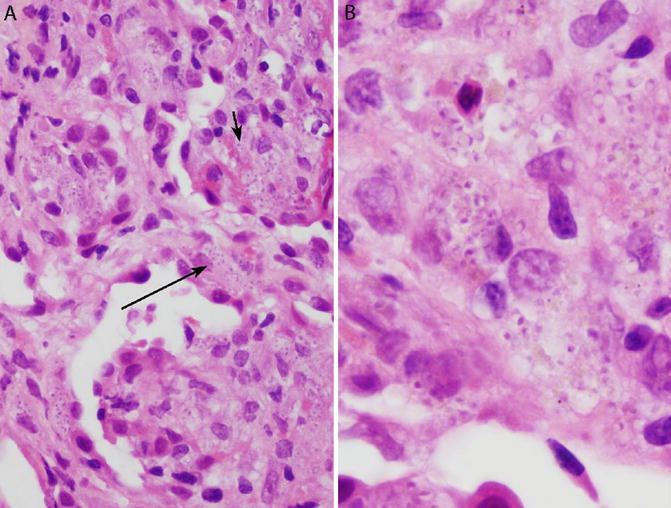

Fig. 6.5
Disseminated histoplasmosis in a transbronchial lung biopsy. The patient was an immunocompromised renal transplant recipient. (a) Numerous histiocytes are present within the airspaces (short arrow) and alveolar septa (long arrow). Organisms are readily visible within the cytoplasm of the histiocytes on H&E. There are no granulomas. (b) Morphologic details are best appreciated under oil immersion. Histoplasma yeasts are round to oval and ring like, with an eccentric purple dot or crescent. This is the form of histoplasmosis that was described by Samuel Darling in 1906
Coccidioidomycosis
Clinical and Radiologic Findings
Coccidioidomycosis is a fungal infection endemic in the southwestern United States including southern Arizona, southern California, New Mexico, Nevada, Utah, and western Texas, as well as in northern Mexico and other areas in Central and South America. Cases are occasionally encountered outside these areas in individuals who visit or move to other states after being infected in an endemic area. In endemic areas, acute exposure causes a flu-like illness known as acute coccidioidomycosis or valley fever (a disease named for the San Joaquin Valley in California). Healed and/or contained infections can cause lung nodules or cavities that mimic malignancy, analogous to histoplasmomas (see “Clinical and Radiologic Findings” section of histoplasmosis). In some series of solitary pulmonary nodules in endemic areas, coccidioidal granulomas are more common than malignancies. Only a minority of patients with nodular/cavitary disease are immunocompromised. The majority have negative results by serology. Thus, a small lung biopsy can be a valuable nonoperative means of establishing a definitive diagnosis.
Histologic Findings in Small Biopsies
Coccidioidomycosis can be diagnosed on needle biopsies [18], although the yield is considered to be poor. Histology is often diagnostic despite negative cultures [3, 24]. In immunocompetent individuals, coccidioidomycosis manifests as necrotizing granulomas with pink (“caseating”) necrosis identical to tuberculomas or histoplasmomas [9] or suppurative necrosis similar to blastomycosis. Eosinophils can be numerous within the necrotic centers of the granulomas as well as in the surrounding lung, and this can be a valuable clue to the diagnosis [25]. However, eosinophils are absent in some cases. Definitive diagnosis rests on the identification of “spherules,” which are large (20–100 μm) round structures with a thick cell wall (Fig. 6.6a, b) [18, 24]. Spherules may be filled with several small yeastlike “endospores” or may be ruptured, fragmented, collapsed, or empty [8]. It has been proposed that the morphologic diagnosis of coccidioidomycosis should require at least one endospore-filled spherule [26]. Both spherules and endospores are visible on H&E, but spherules are easier to recognize because of their large size [9]. They may be found either within the necrotic center of the granuloma or within multinucleated giant cells, even in small biopsies. Budding is absent. Hyphae may occasionally be found. The main organism in the differential diagnosis is Blastomyces, which can usually be distinguished by the presence of broad-based budding and nuclear material. However, in some cases, Coccidioides and Blastomyces cannot be reliably differentiated on histologic grounds.
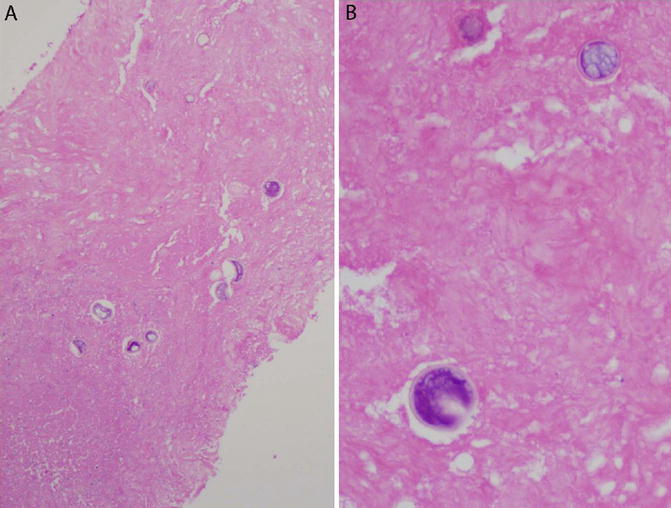

Fig. 6.6
Coccidioides in a necrotizing granuloma. Core needle biopsy of a lung nodule. (a) The main finding in this biopsy is necrosis. Granulomatous inflammation was present elsewhere in the biopsy. Large round fungal organisms are visible on H&E, even at low magnification. (b) Thick-walled Coccidioides spherules, filled with endospores. The presence of these structures is diagnostic
Blastomycosis
Clinical and Radiologic Findings
Blastomycosis is an uncommon fungal infection that usually involves immunocompetent individuals. In North America, most reported cases have been from states bordering the Great Lakes, the St. Lawrence Seaway, the Lower Mississippi valley, and the southeast United States, a geographic distribution similar to histoplasmosis [27]. Cough and fever are common symptoms. Patients may present with a flu-like illness, a bacterial pneumonia-like picture, or acute respiratory distress syndrome (ARDS). Chest imaging can show infiltrates, pneumonia-like consolidation, nodules, or masses with or without cavitation. Extrapulmonary disease can occur, the major sites being the skin, genitourinary tract, and bone [27, 28]. The sensitivity of serology is low. Cultures may take 3–4 weeks to become positive.
Histologic Findings in Small Biopsies
Blastomycosis can be diagnosed in small lung biopsies [21, 28]. Histology can be positive in culture-negative cases [29]. Of the major fungal yeasts that infect the lung, Blastomyces forms the most distinctive granulomas. The granulomas are characteristically “suppurative,” i.e., they have neutrophil-rich centers resembling abscesses. They are frequently mistaken for abscesses and are referred to in the older literature as “pyogranulomatous.” The periphery of the granulomas is composed of epithelioid histiocytes and variable numbers of multinucleated giant cells. Organisms can be found both in the suppurative center and the granulomatous rim [2, 21]. Blastomyces yeasts are large (8–15 μm) with a thick double-contoured cell wall. A characteristic feature is the presence of basophilic nuclear material. The organisms are visible on H&E as well as GMS. Although GMS highlights far more organisms than are apparent on H&E, it also obscures the nuclear material. Blastomyces characteristically forms a single broad-based bud, a key feature in the differential diagnosis with other morphologically similar fungal yeasts, including Cryptococcus and Coccidioides [1].
Aspergillosis
Clinical and Radiologic Findings
Fungal hyphae such as Aspergillus usually do not cause granulomatous inflammation in the lung. However, a minority of pulmonary infections with these fungi are associated with a granulomatous response, which appears to be a marker of limited tissue invasion. The best-known clinical entity in which granulomatous inflammation occurs is chronic necrotizing pulmonary aspergillosis, also known as semi-invasive aspergillosis [30, 31]. The condition was described as a chronic progressive infection characterized by persistent or enlarging lung cavities in individuals without preexisting cavitary lung disease. Subsequent studies have shown a predilection for men and individuals with emphysema or compromised immunity.
Histologic Findings in Small Biopsies
Most instances of this entity are characterized by necrotizing granulomatous inflammation similar to tuberculosis or histoplasmosis [32]. The diagnosis rests on demonstration of fungal hyphae within the granulomas, usually in the cavitary center. Aspergillus cannot be reliably differentiated from other morphologically similar fungal hyphae without microbiologic confirmation.
Wegener’s Granulomatosis (Granulomatosis with Polyangiitis/ GPA)
Clinical and Radiologic Findings
Wegener’s granulomatosis is a form of vasculitis that classically affects the lungs, kidneys, and upper respiratory tract. It is the most common type of vasculitis seen in lung biopsies. Patients usually present with cough, hemoptysis, dyspnea, renal failure, sinusitis, and/or epistaxis. Radiographic findings include bilateral cavitary nodules or infiltrates. Some patients present with clinical and radiologic features of diffuse alveolar hemorrhage. Most cases are positive for anti-neutrophil cytoplasmic antibodies (ANCA). Positive immunofluorescence for cytoplasmic ANCA (c-ANCA) with antibodies against proteinase 3 has high specificity for Wegener’s granulomatosis. However, it is well documented that Wegener’s granulomatosis can present with localized pulmonary involvement and that such cases are significantly more likely to be ANCA negative than those with multisystem disease. It is such cases that cause the greatest diagnostic difficulties. Radiologically, these cases may present with bilateral lung nodules. However, Wegener’s granulomatosis presenting as a solitary lung nodule has also been described [33]. Biopsies are most often performed when the clinical setting is unusual. Pathologists must therefore be prepared to recognize the histologic features of Wegener’s granulomatosis in atypical settings and should not be deterred by the absence of multisystem involvement or a negative ANCA. Patients with localized pulmonary necrotizing granulomas can subsequently develop extrapulmonary vasculitis and become ANCA positive on follow-up [10].
Histologic Findings in Small Biopsies
The classic histologic findings of Wegener’s granulomatosis are necrotizing granulomatous inflammation and necrotizing vasculitis. This combination is required for a definitive pathologic diagnosis of Wegener’s granulomatosis. Unfortu-nately, it is often difficult or impossible to demonstrate since the affected vessels may be completely destroyed. The necrosis of Wegener’s granulomatosis is often described as “dirty” due to its basophilic hue, which is caused by nuclear debris derived from necrotic neutrophils (Fig. 6.7a, b). Suppurative (neutrophil rich) necrosis may also be present. “Geographic” necrosis, characterized by necrotic areas with irregular contours, cannot be appreciated in a small biopsy. Tiny microabscess-like granulomas with suppurative centers may be a clue to the diagnosis. They consist of aggregates of a few multinucleated giant cells surrounding a minute cluster of neutrophils [34]. Multinucleated giant cells with prominent hyperchromatic nuclei may accompany the necrotizing granulomas. When demonstrable, necrotizing vasculitis manifests as a mixed neutrophilic–histiocytic infiltrate within vessel walls, often accompanied by small, subtle foci of fibrinoid necrosis. The infiltrate frequently involves only one side of the affected vessel (“eccentric vasculitis”) (Fig. 6.7a, b). Karyorrhectic nuclear debris is often present. Findings that should not be misinterpreted as necrotizing vasculitis include vascular inflammation comprised entirely of lymphocytes and plasma cells, granulomas in the vessel wall, and necrotic vessels within areas of parenchymal necrosis. In virtually all cases, there is a background of severe nonspecific acute and chronic inflammation containing neutrophils, lymphocytes, and plasma cells. Organizing pneumonia (plugs of fibroblasts within airspaces) may be present. The presence of hemosiderin-laden macrophages in and around the inflammatory process is another clue to the correct diagnosis.
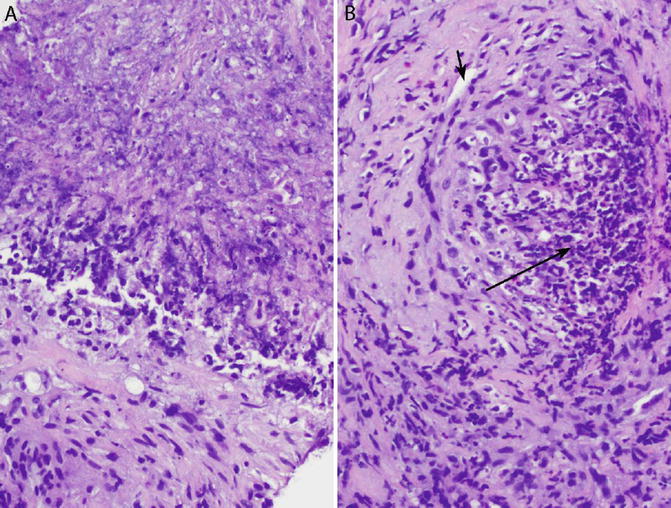

Fig. 6.7
Granulomatosis with polyangiitis (Wegener’s granulomatosis). Core needle biopsy. (a) “Dirty” necrosis is basophilic due to abundant nuclear debris. (b) Necrotizing vasculitis. The wall of this medium-sized blood vessel is focally infiltrated and destroyed by an inflammatory infiltrate consisting of neutrophils and histiocytes (long arrow). Note that the left side of the vessel is relatively spared (short arrow is in lumen). This type of “eccentric vasculitis” is typical of Wegener’s granulomatosis
Wegener’s granulomatosis occasionally presents with diffuse pulmonary hemorrhage, the histologic correlate of which is “capillaritis,” a destructive vasculitis affecting alveolar septal capillaries [35–37]. It is characterized by a mixed neutrophilic–histiocytic inflammatory infiltrate that destroys alveolar septa and is accompanied by intra-alveolar hemorrhage consisting of variable combinations of fresh blood (red blood cells), fibrin, and hemosiderin-laden macrophages. The neutrophils invariably spill into the adjacent airspaces, causing acute bronchopneumonia to enter the differential diagnosis.
Transbronchial lung biopsies may occasionally show suggestive but nonspecific findings such as acute inflammation mixed with granulomatous inflammation or granulomas with dirty necrosis [38]. In such cases, a definitive diagnosis cannot be made by histology alone and requires correlation with ANCA serologies.
Churg–Strauss Syndrome (Eosinophilic Granulomatosis with Polyangiitis/EGPA)
Clinical and Radiologic Findings
Churg–Strauss syndrome is a rare disease of asthmatic individuals characterized by tissue and blood eosinophilia, granulomatous inflammation, and vasculitis. Patients typically present with fever, weight loss, striking peripheral (blood) eosinophilia, and transient or migratory pulmonary infiltrates. Imaging features include ground-glass opacities, centrilobular nodules, and airspace consolidation [39]. Many patients have extrapulmonary manifestations such as cutaneous lesions or neuropathy. Perinuclear ANCA (p-ANCA) is positive in approximately two-thirds. Many cases are diagnosed without biopsy or with a biopsy of a site other than the lung.
Histologic Findings in Small Biopsies
The most consistent histologic feature of Churg–Strauss syndrome is the presence of increased numbers of eosinophils within the affected tissues. The full spectrum of diagnostic findings, which consists of a combination of necrotizing vasculitis, granulomatous inflammation with eosinophils, and eosinophilic pneumonia [4], is rarely seen, especially in transbronchial biopsies [40]. However, pathologists should recognize that any of these features—especially in combination with numerous eosinophils—raises the possibility of Churg–Strauss syndrome in the appropriate clinical context.
Rheumatoid Nodule
Clinical and Radiologic Findings
Rheumatoid nodules typically present as multiple pulmonary nodules in patients with known rheumatoid arthritis [10]. Most patients are seropositive at the time of diagnosis, and some have concomitant subcutaneous rheumatoid nodules.
Histologic Findings in Small Biopsies
It is impossible to make this diagnosis with certainty on a small biopsy since it is partially a diagnosis of exclusion that rests on the finding of necrotizing granulomatous inflammation without evidence of organisms or necrotizing vasculitis in patients with known rheumatoid arthritis. The histologic findings are not distinctive. The role of pathology is to exclude alternative possibilities, the most important being infection and Wegener’s granulomatosis.
Rare Infectious Causes of Pulmonary Granulomas
A handful of other organisms cause granulomatous inflammation in the lungs, but are very unlikely to be seen in routine practice, especially in small lung biopsies. These include fungi such as Sporothrix, which typically causes suppurative granulomas in the skin but can also involve the lungs [41], and Pneumocystis, which usually causes a frothy intra-alveolar exudate but can rarely be associated with granulomatous inflammation [22], and parasites such as Dirofilaria, which causes granulomas with infarct-like necrosis due to embolization from the right side of the heart into pulmonary arteries [42].
Necrotizing Granulomas of Unknown Etiology
It is common for necrotizing granulomas to be found in lung specimens with no histologic evidence of a definite etiology, infectious or otherwise. This is especially true in small biopsies, which do not sample the entirety of the lesion. Diagnostic findings such as organisms or vasculitis are therefore easily missed. The problem is greater than mere sampling error. Studies have demonstrated that even when entire necrotizing granulomas are resected surgically, an etiology cannot be found in a significant percentage of cases [7, 10]. There is substantial evidence to support the notion that such lesions are “burnt-out” mycobacterial or fungal infections in which organisms have been destroyed or removed by the granulomatous inflammatory response.
What can pathologists do if the etiology of a granulomatous process is not apparent and special stains are negative? [4, 43] Of the several steps that can reveal an etiology in such cases, the most important one is to carefully reexamine the histologic findings, especially the GMS-stained slides. Waiting for results of cultures may be productive since some organisms, especially mycobacteria, grow in cultures even though special stains may be negative. Finally, clinical information, specifically radiologic findings, results of ANCA tests, and a history of rheumatoid arthritis, can help in making a specific diagnosis in some cases. The best way to communicate these issues to the clinician is in a comment, instead of encumbering the diagnostic line with a wordy description. The diagnosis can then simply be “necrotizing granulomatous inflammation (see comment).”
Causes of Non-necrotizing Granulomatous Inflammation
Sarcoidosis
Clinical and Radiologic Findings
The diagnosis of sarcoidosis depends on a combination of clinical context and pathologic findings [44]. Yet, details of clinical and radiologic findings are rarely, if ever, provided to pathologists at the time of the initial diagnosis. However, pathologists with access to electronic medical records should be familiar with key findings that indicate whether the clinical context is appropriate for sarcoidosis. Typical patients with sarcoidosis are young individuals of either gender, with a peak in the 30s and 40s [45]. Cases have been well documented in the elderly but are infrequent [46]. The most common radiologic finding is bilateral hilar or mediastinal adenopathy. Bilateral hilar adenopathy in an asymptomatic young person is considered highly suspicious for sarcoidosis [44]. In the lungs, radiologic findings may be absent or may consist of bilateral infiltrates or nodules. Patients may be asymptomatic, the radiologic findings having been discovered incidentally, or they may present with cough and dyspnea. Sarcoidosis is a multisystem disease and may involve virtually any other organ. Patients can present with a wide variety of symptoms referable to involvement of these sites, such as erythema nodosum (skin) and uveitis (eye).
Clinical findings that argue against sarcoidosis include unilateral infiltrates, a unilateral mass, bronchiectasis, predominant airspace opacities, lack of response to steroids, and absence of hilar or mediastinal adenopathy. Needless to say, positive cultures for mycobacteria or fungi exclude the diagnosis. Unfortunately, culture results are seldom available at the time of biopsy [47]. Therefore, the pathologic diagnosis, and often the clinical diagnosis, is usually made before an infection can be definitively excluded.
Histologic Findings in Small Biopsies
In western countries, sarcoidosis is by far the most common cause of granulomas in transbronchial biopsies [3]. Endobronchial ultrasound (EBUS) with transbronchial needle aspiration is increasingly used to demonstrate granulomas in hilar lymph nodes [48]. The histologic hallmark of sarcoidosis is the non-necrotizing (“noncaseating”) granuloma [1, 20]. However, non-necrotizing granulomas are also found in other lung diseases [47], making it important for pathologists to recognize histologic features that are consistent with sarcoidosis and those that exclude or argue against the diagnosis [1]. Pathologists are well served to remember that one of their key responsibilities is the exclusion of alternative diagnoses, including infection, malignancy, and other causes of granulomatous lung disease.
In transbronchial biopsies, the granulomas of sarcoidosis are found in the bronchial mucosa or in the alveolar septa (interstitium) (Figs. 6.8a, b and 6.9a, b) [4, 47]. In classic cases, they are plump, well formed, and surrounded by layers of concentric hyalinized fibrosis (Fig. 6.8a, b). Multiple small granulomas may fuse into larger nodules. Necrosis can occur but in most cases is minimal, tends to have a degenerated or fibrinoid quality, and usually involves only a few granulomas [49]. Nonspecific inclusions of various kinds, including pink spider-like structures (asteroid bodies), basophilic concentric calcifications (Schaumann bodies), and calcium oxalate crystals, may be found within the granulomas (Fig. 6.9a, b) [1, 47, 49]. These are thought to be endogenous products of macrophage metabolism and are not specific for sarcoidosis. The classic lymphangitic (lymphatic) distribution of sarcoid granulomas—along the pleura, interlobular septa, and bronchovascular bundles—is a valuable diagnostic feature in surgical lung biopsies but for obvious reasons cannot be appreciated in transbronchial biopsies. Special stains for microorganisms should be performed in all cases. Histologic features that argue against sarcoidosis—and suggest an alternative diagnosis—include extensive necrosis, suppurative or “dirty necrosis,” organizing pneumonia (fibroblast plugs within the airspaces), and foreign particles (vegetable particles, talc, microcrystalline cellulose, or crospovidone) [4]. An important histologic feature of sarcoidosis is that chronic inflammation, when present, is localized to the area surrounding the granulomas (Fig. 6.9a, b). The lung away from the granulomas is normal. This feature helps to differentiate sarcoidosis from hypersensitivity pneumonitis, in which interstitial chronic inflammation is found even in lung parenchyma away from granulomas [1, 50, 51]. Needless to say, identification of mycobacterial or fungal organisms on H&E or special stains excludes sarcoidosis. On the other hand, negative special stains do not “confirm” a diagnosis of sarcoidosis, since these can be negative in infections [47].
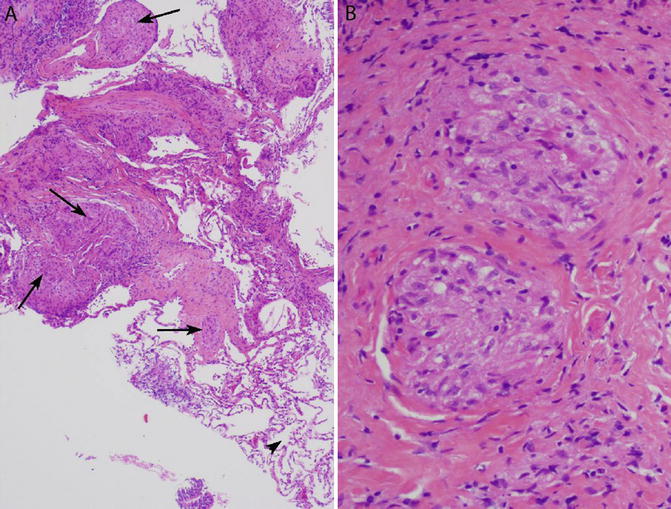
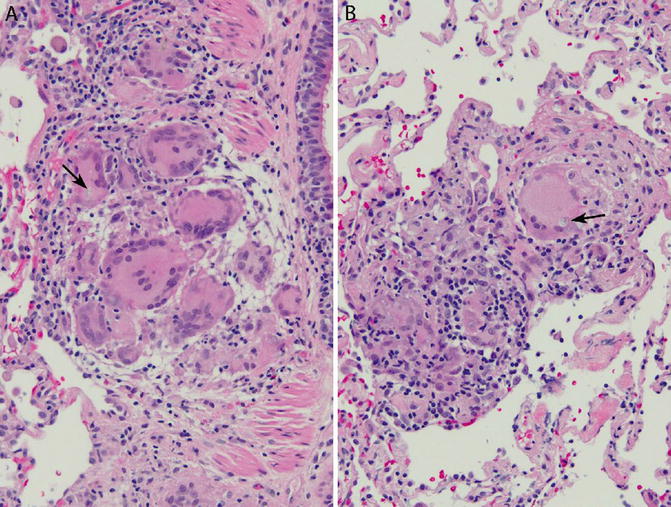

Fig. 6.8
Non-necrotizing granulomas consistent with sarcoidosis in a transbronchial lung biopsy. (a) Multiple non-necrotizing granulomas are present within the interstitium (arrows). An important finding is the presence of normal lung away from the granulomas (arrowhead, bottom right). (b) Same biopsy, high magnification. The granulomas are well formed and surrounded by hyalinized fibrosis

Fig. 6.9




Non-necrotizing granulomas consistent with sarcoidosis. Transbronchial lung biopsy. (a) Non-necrotizing granuloma within bronchiolar wall. An inclusion is present within a giant cell (arrow). (b) Non-necrotizing granuloma within the interstitium. The granuloma is small and ill defined. A giant cell in the granuloma contains an inclusion (arrow). There is no hyalinized fibrosis. Lung parenchyma away from the granuloma is normal. Although the findings are consistent with sarcoidosis, they are less typical than the case illustrated in Fig. 6.8. Also compare with hypersensitivity pneumonitis (Figs. 6.10, 6.11, and 6.12)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


