Fig. 1.1
Approximate range of odds ratios for risk factors for symptomatic peripheral arterial disease (Reprinted from Norgren et al. with permission from Elsevier [9]
Hyperhomocysteinemia
Hyperhomocysteinemia may be an independent risk factor for atherosclerosis as it is detected in about 30 % of young patients with PAD compared to 1 % in the general population [10].
Inflammatory Markers/C-Reactive Protein
Recent studies have shown that C-reactive protein (CRP) is higher in asymptomatic subjects who developed PAD in the subsequent 5 years, compared to an age-matched control group who remained asymptomatic [11].
Chronic Renal Insufficiency
PAD has been associated with chronic renal insufficiency.
Hypercoagulable States
Hyperviscosity and raised hematocrit have been detected in patients with PAD, which may be a consequence of smoking. Increased plasma fibrinogen levels have also been associated with PAD in some studies [6].
Ethnicity
PAD defined as an ABI of ≤0.9 was more common in non-Hispanic blacks (7.8 %) than in whites (4.4 %) [12].
Noninvasive Vascular Laboratory Exams
Cerebrovascular Noninvasive Vascular Testing
Carotid duplex ultrasound remains the initial exam of choice for evaluating patients with suspected cerebrovascular disease.
Indications
Common indications for carotid duplex sonography include a cervical bruit in an asymptomatic patient and the presence of symptoms suggesting a hemispheric or retinal TIA or stroke [13, 14]. Carotid duplex exams can provide a stroke risk assessment for patients with coronary disease and PAD as well as patients with multiple atherosclerotic risk factors. Other less common indications for carotid duplex sonography include intraoperative assessment during carotid surgery or stenting. Patients with posterior circulation symptoms which typically involve exertional lightheadedness or impaired vision associated with upper extremity exertion also warrant a carotid duplex exam.
Follow-Up After Carotid Intervention/Surveillance
Patients with atherosclerotic risk factors and an initial duplex exam demonstrating less than 50 % stenosis of the internal carotid artery (ICA) can be reevaluated at 1–2-year intervals. Stenosis between 50 and 69 % should be followed at 6–12 month intervals whereas severe stenosis greater than 70 % should be considered for immediate intervention or surveillance every 6 months depending on the clinical scenario.
Limitations
The diagnostic accuracy of a carotid duplex exam depends on adequate sonographic visualization of the carotid arteries. Calcification creates acoustic shadows which impede ultrasound waves and prevent interrogation of blood flow velocities within the calcified arteries. Other conditions which prevent sonographic visualization include a cervical hematoma, soft tissue edema, the presence of sutures or skin staples, and exceptionally deep or tortuous vessels in patients with large necks.
A carotid duplex exam indirectly determines the severity of stenosis by measuring the blood flow velocity which is derived from the frequency shift using the Doppler equation. The accuracy of the exam therefore depends on measuring the true blood flow velocity with a known angle of insonation (usually 60°). Underestimating disease can occur in patients with long, smooth plaque formation which does not cause the accelerated, turbulent flow pattern usually associated with a focal, segmental stenosis. Failure to appreciate low-intensity echoes of soft plaque or using an inappropriate Doppler angle can also underestimate the degree of stenosis. Overestimating the severity of stenosis can occur when an artifact is mistaken for a carotid plaque and when physiologically accelerated flow is inappropriately attributed to stenosis. The presence of vessel kinking or tortuosity, significant contralateral stenosis or occlusion, and cardiac dysrhythmias makes it more difficult to evaluate the flow spectra. Large-caliber vessels generally have lower flow velocities compared to smaller-caliber vessels at the same flow intensity. Therefore, blood flow in a wide carotid sinus can be easily disturbed and this physiologic turbulence could be falsely attributed to arterial stenosis.
Essentials of Examinations/Interpretation of Results
A duplex examination should provide detailed information of the extracranial carotid arteries. Grayscale (B-mode) imaging can characterize the plaque morphology within the common, internal, and external carotid arteries. Peak systolic and end diastolic velocities should be recorded at several points along the course of the common and internal carotid arteries as well as at least one point in the external carotid artery. The ratio of peak systolic velocity (PSV) in the internal carotid artery to the common carotid artery provides additional information to measure stenosis. Unfortunately many vascular laboratories do not meet the exam requirements and quality assurance guidelines required for accreditation by the Intersocietal Accreditation Commission (IAC). Exam reports from unaccredited labs often lack reliability and may limit the physician’s ability to rely solely on this modality prior to carotid surgery. In 2003 Grant et al. reported consensus velocity criteria for duplex ultrasonography in an attempt to establish a more uniform method of classifying carotid stenosis (Tables 1.2 and 1.3) [15, 16]. Figures 1.2 and 1.3 show duplex ultrasound images of a normal carotid artery and severe stenosis of the ICA, respectively.
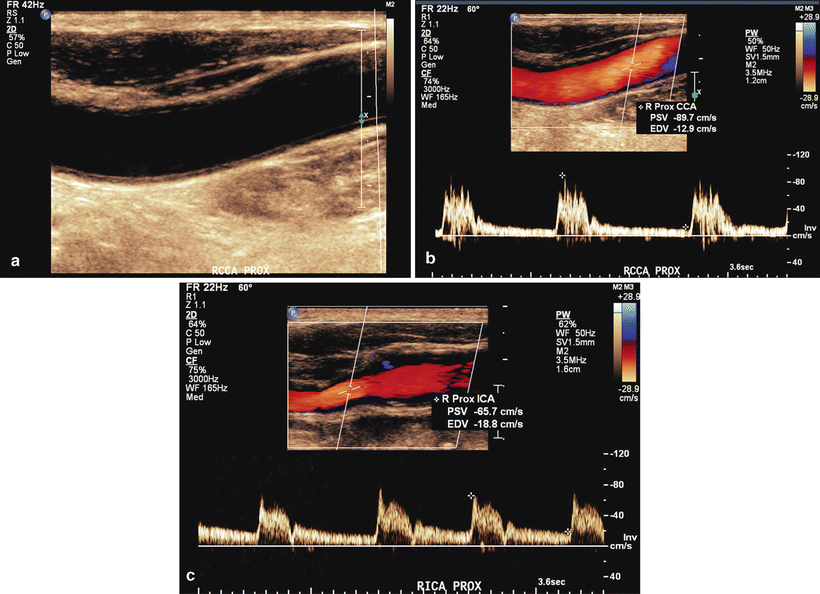
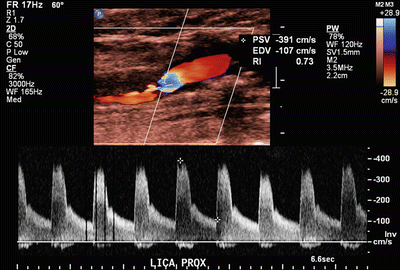

Fig. 1.2
(a) B-mode image of proximal common carotid artery. (b) Color flow of proximal common carotid artery. (c) Duplex image of normal proximal internal carotid artery: PSV of 66 cm/s and EDV of 19 cm/s, no plaquing

Fig. 1.3
Duplex ultrasound of severe internal carotid artery stenosis: PSV of 391 cm/s and EDV of 107 cm/s with significant carotid plaquing
Table 1.1
Classification of peripheral arterial disease (PAD): Rutherford categories
Grade | Category | Clinical description |
|---|---|---|
0 | 0 | Asymptomatic |
I | 1 | Mild claudication |
I | 2 | Moderate claudication |
I | 3 | Severe claudication |
II | 4 | Ischemic rest pain |
III | 5 | Minor tissue loss |
III | 6 | Major tissue loss |
Table 1.2
Primary parameters for carotid duplex sonography
Duplex criteria | Interpretation |
|---|---|
ICA PSV of <125 cm/s with no visible plaques | Normal carotid |
ICA PSV of <125 cm/s with visible plaque of less than 50 % diameter reduction | Less than 50 % stenosis |
ICA PSV range of 125–230 cm/s with a visible plaque estimate of greater than 50 % diameter reduction | 50–69 % stenosis |
ICA PSV of greater than 230 cm/s and a plaque estimate of greater than 50 % diameter reduction | Greater than 70 % stenosis but less than near occlusion |
High, low, or undetectable PSV’s with visible plaques, variable systolic ratio | Near occlusion |
Undetectable flow with visible plaque, no detectable lumen | Total occlusion |
Table 1.3
Other parameters recommended for use only in borderline data
Duplex criteria | Interpretation |
|---|---|
ICA/CCA PSV ratio of less than 2 and an ICA EDV of less than 40 cm/s | Normal carotid |
ICA/CCA PSV ratio of 2–4 and an ICA EDV of 40–100 cm/s | 50–69 % stenosis |
ICA/CCA ratio of greater than 4 and an ICA EDV greater than 100 cm/s | Greater than 70 % stenosis to near occlusion |
Abdominal Aortic Ultrasound
The infrarenal abdominal aorta is the most common site of aneurysmal degeneration of the aorta. An abdominal aortic ultrasound can function both as a screening exam for AAA and as a surveillance imaging modality for patients with small AAA’s (Fig. 1.4).
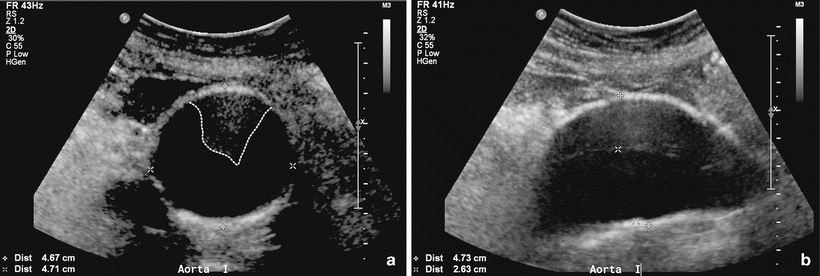

Fig. 1.4
Ultrasound of native aorta showing abdominal aortic aneurysm, (a) transverse view – notice intraluminal thrombus (dotted line), (b) sagittal view
Indications
An aortic ultrasound should be used to evaluate patients with a pulsatile abdominal mass. Patients with a history or presence of a popliteal artery aneurysm should also be evaluated for an AAA because of the strong association between peripheral and aortic aneurysms. Appropriate candidates to undergo a screening abdominal aortic ultrasound exam include men or women older than 60 who have a first-degree relative with an AAA and men older than 65 who have ever smoked cigarettes.
Limitations
Since ultrasound waves do not propagate well through air, the presence of excessive bowel gas prevents visualization of the aorta and renders the exam non-diagnostic. In morbidly obese patients, the depth of the abdominal aorta can exceed the penetration of even the lowest frequency ultrasound probe. Technically challenging ultrasound exams due to patient body habitus or previous abdominal surgery can compromise the spatial resolution of the sonographic images making it difficult to characterize aneurysm morphology. Ultrasound imaging may fail to identify saccular aneurysms which have an unpredictable natural history compared to the more commonly encountered fusiform aneurysms.
Surveillance
The Society for Vascular Surgery recommends the following schedule for abdominal aortic ultrasound examinations in patients with an AAA: every 6 months for aneurysms with maximum diameter of 4.5–5.4 cm, annually for aneurysms measuring 3.5–4.4 cm, every 3 years for 3–3.4 cm AAAs, and in 5 years if the aortic diameter is 2.6–2.9 cm. Aneurysms exceeding 5.5 cm in diameter should be evaluated with computed tomography to plan for surgical or endovascular repair.
Essentials of Evaluation/Interpretation
Grayscale images with a transverse view of the aorta allow for measurement of the maximal aneurysm diameter. Ultrasound measurements of the aorta have proven to be accurate and reproducible; the results usually come within 5 mm of measurements taken using computed tomography which is considered the gold standard [17]. It is important to clearly interpret the ultrasound exam report since some laboratories provide the length of the aneurismal segment in addition to its maximal diameter. The diameter and rate of growth are the clinically important data points in the assessment of infrarenal AAAs. Occasionally, urgent vascular surgery referrals are made for 8 cm aneurysms that turn out to be only 4 cm in diameter but 8 cm in length.
Ankle Brachial Index
The ankle brachial index (ABI) is the screening modality of choice for diagnosing patients with PAD. An ABI can be performed in virtually any clinical setting using a manual sphygmomanometer and a handheld continuous wave Doppler. The patient should be in the supine position. With the blood pressure cuff placed above the patient’s ankle, the Doppler probe is used to locate the dorsal pedal or posterior tibial pulse. The blood pressure cuff is then inflated until the Doppler signal is no longer audible. The pressure at which the Doppler signal returns as the cuff slowly deflates is the systolic ankle pressure. The process is repeated for the remaining pedal pulse and then on the other leg. The brachial pressure in both arms should be measured using a similar technique with the blood pressure cuff around the upper arm and Doppler interrogation of the brachial, radial, or ulnar pulse. (The measured pressure is determined by cuff location, not the Doppler site.) The ABI is the ratio of the higher of the ankle pressures (posterior tibial or dorsal pedal) of the ipsilateral lower extremity compared to the highest brachial pressure (either right or left arm). The ABI is a reliable predictor of long-term survival and future risk of limb loss. Men with an ABI of less than 0.9 had an 18 % cardiovascular-related mortality rate at 10-year follow-up compared to a 4 % mortality rate for men with a normal ABI [18, 19].
In addition to the ABI, segmental Doppler pressures of the lower extremity, including high thigh, low thigh (above knee), below knee, and ankle pressures can be measured using appropriately placed blood pressure cuffs (Fig. 1.5). These exams are usually performed in the noninvasive vascular lab and provide more precise localization of occlusive lesions in the lower limb [20]. Note that many vascular labs use a single wide cuff on the thigh instead of the two cuffs depicted in Fig. 1.5.
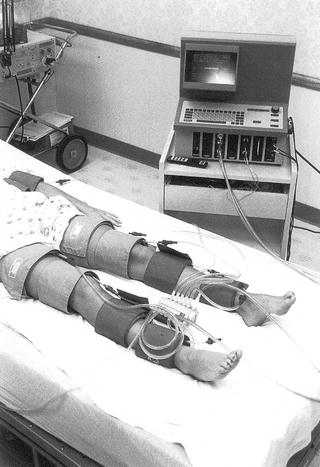

Fig. 1.5
Technique for measuring the segmental Doppler pressures using the four-cuff method
Indications
A screening ABI should be considered in patients with diminished or absent peripheral pulses and in patients with symptomatic PAD. Patients with diabetes and current or previous smokers over the age of 50 should also have a screening ABI.
Limitations
Calcified arteries can render the ABI inaccurate by falsely elevating the ankle pressure or making the tibial arteries noncompressible. These changes occur more frequently in patients with long-standing diabetes mellitus. In patients with noncompressible ABIs, the Doppler pressure in the toe accurately reflects arterial perfusion since calcification usually spares the small digital blood vessels. Toe pressures may also be a reasonable alternative in patients who cannot have an ABI because of the presence lower leg wounds which prevent application of a blood pressure cuff. Most noninvasive vascular laboratories have appropriately sized blood pressure cuffs to measure the toe pressure.
Interpretation of Results
Normally the systolic ankle pressure equals or slightly exceeds the systolic brachial pressure. Patients with normal lower extremity arterial pressure therefore have an ABI of 1.0–1.2. PAD is defined as an ABI of less than 0.9 and the severity of PAD increases as the ABI decreases (Table 1.4). Patients with significant medial calcinosis often have a falsely elevated ABI which can signify severe PAD and predict poor long-term outcomes. Segmental blood pressure measurements indicate the presence of significant arterial disease if they demonstrate a pressure decrease of 30 mmHg or greater between adjacent segments, e.g., high thigh and above knee pressures (for superficial femoral occlusion, Fig. 1.6) or above knee and below knee pressures (for popliteal occlusion). Toe pressure and the calculated toe brachial index can predict wound healing following digital and foot amputations.
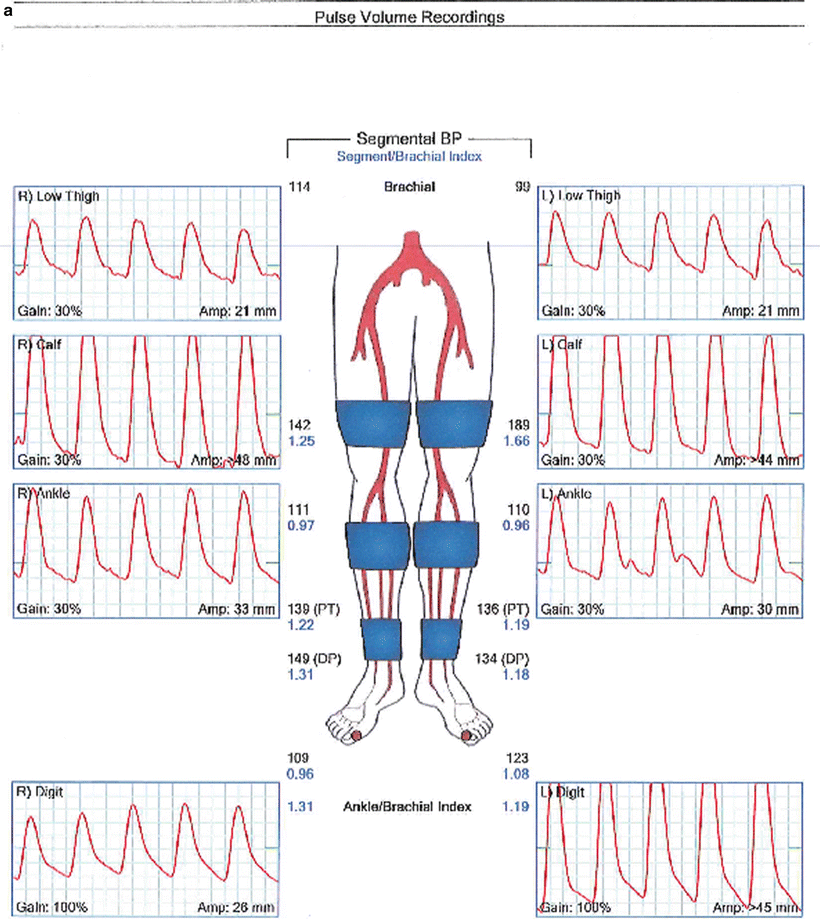
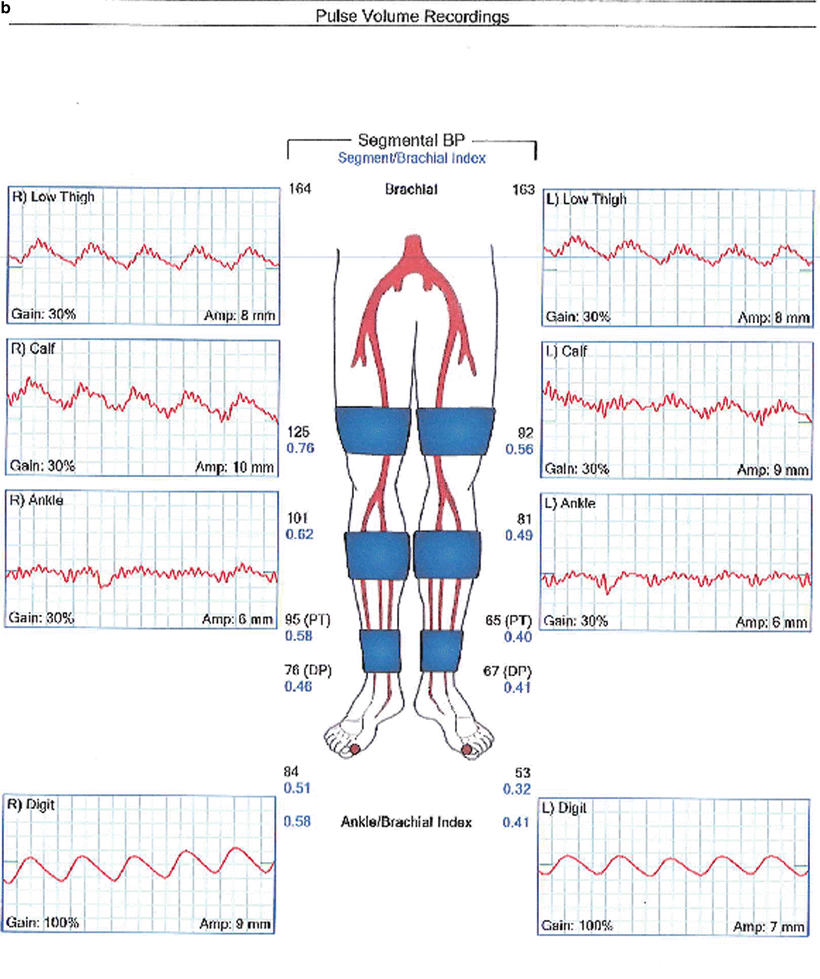


Fig. 1.6
Segmental systolic limb pressures and pressure volume recordings in a patient with normal lower extremity arterial perfusion (a) and in a patient with severe aortoiliac arterial occlusive disease (b)
Table 1.4
Ankle brachial index (ABI)
ABI | Interpretation |
|---|---|
1.0–1.2 | Normal |
0.7–.09 | Mild PAD |
0.5–.07 | Moderate PAD |
0.3–0.5 | Severe PAD |
<0.3 | Critical limb ischemia |
Duplex Ultrasound Exam of the Lower Extremity
An arterial duplex exam is a unique diagnostic tool that provides both anatomic and physiologic information. The exam involves grayscale imaging and blood flow velocity measurements along the entire course of the lower extremity. The level of detail provided by an arterial duplex can localize the diseased arterial segments and differentiate between stenosis and complete occlusion.
Indications
An arterial duplex exam is a valuable diagnostic tool to evaluate patients with symptomatic PAD including those with claudication, ischemic rest pain, or tissue loss. Patients with a leg or foot infection who do not have palpable pulses should also be considered for lower extremity arterial duplex evaluation. Since an arterial duplex does not require the use of intravascular contrast, this imaging modality is useful for evaluating patients with coexisting renal impairment. Arterial duplex exams also play an important role in the surveillance of patients with lower extremity bypasses. Careful follow-up using arterial duplex scans can improve the long-term patency of infrainguinal bypasses by detecting areas of progressive stenosis thereby allowing for therapeutic intervention before the bypass graft completely occludes.
Limitations
Arterial calcifications reflect ultrasound waves and may limit the ability to detect and measure the blood flow velocity. Patient factors such as severe lower extremity edema and obese body habitus increase the technical difficulty and decrease the accuracy of a lower extremity arterial duplex exam. Like other noninvasive ultrasound exams, the overall quality of an arterial duplex often depends on the skill and persistence of the sonographer.
Arterial duplex ultrasonography is an extremely sensitive and labor-intensive exam that should not be used to screen patients for the presence of PAD. The evaluation of patients suspected of having PAD should begin with the history and physical followed by an ABI. Arterial duplex should be reserved for patients with an unclear diagnosis or in patients in whom an intervention is being considered.
Duplex Ultrasound Interpretation in Patients with PAD
The results of an arterial duplex exam are usually expressed as peak-systolic velocity (PSV) and velocity ratio (VR) which is calculated by dividing the PSV at the stenosis by the PSV just proximal to the stenosis. Khan et al. defined greater than 70 % stenosis in the native femoropopliteal arteries as a PSV exceeding 200 cm/s and a VR greater than 2.5 [21]. Table 1.5 lists the velocity criteria proposed by Armstrong and Bandyk for de novo femoropopliteal arterial lesions [22].
Table 1.5
Duplex-specific velocity criteria for femoropopliteal stenosis
PSV | VR | Degree of stenosis |
|---|---|---|
<200 cm/s | <2 | Less than 50 % |
200–300 cm/s | 2–4 | 50–75 % |
>300 cm/s | >4 | Greater than 75 % |
No flow | – | Complete occlusion |
The presence of a proximal stenotic or occlusive lesion can reduce downstream blood flow velocity. Preserving the accuracy and sensitivity of the arterial duplex exam may therefore require adjusting the velocity criteria for patients with multilevel occlusive disease. Figure 1.7 shows a duplex ultrasound of a hemodynamically significant stenosis in the superficial femoral artery.
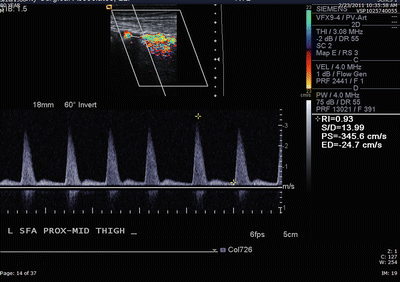

Fig. 1.7
Duplex scan of mid-thigh superficial femoral artery stenosis in a patient with calf claudication. At rest, a triphasic waveform was recorded proximal and at a focal stenosis with a PSV of 441 cm/s and VR of 8.4 (criteria indicating a >75 % diameter reducing stenosis [22])
Duplex Vein Mapping
Indication
The K-DOQI guidelines for arteriovenous access recommend preoperative duplex vein mapping for all patients who require hemodialysis access surgery. Duplex vein mapping increases the creation of native arteriovenous fistulas by detecting adequate caliber superficial veins in the upper extremity [23]. It is not uncommon to have a patient referred with a previous failed arteriovenous graft who still has suitable veins for autogenous fistula creation detected by duplex vein mapping. Preoperative duplex vein mapping of the saphenous veins (great and small) can be useful in patients who require lower extremity arterial bypass.
Limitations
The fluid status of the patient and the ambient room temperature can influence the diameter of superficial veins. Performing a duplex vein mapping on a dehydrated patient in a cool room can significantly underestimate the true caliber of the veins. In obese patients the large size of the arm can limit the ability to precisely measure vein diameter.
Essentials of Evaluation/Interpretation of Results
Upper extremity vein mapping should be performed in a warm room on well-hydrated patients. The exam begins by evaluating the patency of the deep and superficial veins along their entire course in the upper arm and forearm. Patent veins demonstrate complete compressibility with gentle pressure from the ultrasound probe. Most vascular laboratories perform superficial vein diameter measurements with a tourniquet in place on the proximal upper arm. The anterior-posterior and transverse diameters of both the cephalic and basilic veins from the wrist to axilla should be recorded in chart form to enhance clarity. Superficial veins with a diameter of 3 mm or more have the best chance of maturing into a functional arteriovenous fistula. Evaluating vein diameters in the more proximal upper extremity in addition to recording the vein caliber at the proposed anastomotic site can identify sclerotic vein segments that could impede fistula maturation. The basilic vein in the upper arm always requires ultrasound evaluation since its location deep to the fascia prevents visual inspection.
A thorough preoperative exam for patients requiring hemodialysis access includes bilateral brachial blood pressure measurement and waveform analysis of the axillary, brachial, radial, and ulnar arteries. A greater than 20 mm Hg difference in upper extremity blood pressures warrants using the extremity with the higher pressure or performing preoperative angiography to assess for arterial occlusive lesions. Waveform analysis may indicate the presence of atherosclerotic disease in the upper extremity which can predispose the patient to develop hemodynamic steal symptoms after AV fistula creation. Patients with a history or physical exam findings which suggest central venous stenosis should be considered for preoperative venography to evaluate the central veins prior to access creation.
Venous Duplex Ultrasound for Endovascular Therapy
Endovenous ablation of the great saphenous vein has become one of the most common vascular procedures performed in the United States. Instead of surgically stripping an incompetent great saphenous vein, an endovenous ablation procedure uses percutaneous access to heat the inside of the vein. This intraluminal heat denatures the endothelium resulting in permanent occlusion of the vein. Duplex ultrasound plays a central role in both the preparation for and the performance of endovenous ablation procedures. Prior to an intervention, venous duplex sonography can evaluate for the presence of superficial venous reflux that is amenable to endovenous treatment. During the endovenous procedure, ultrasound provides imaging guidance for percutaneous access, placement of the ablation catheter, and infusion of tumescent anesthesia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


