Diabetes and Metabolic Syndrome
Emily D. Szmuilowicz
Merri Pendergrass
Diabetes mellitus and the “metabolic syndrome” are among the most challenging problems facing health care providers today. Over 18 million Americans, or about 9% of the adult population, are currently estimated to have diabetes (1). Approximately 50 million Americans, or 24% of adults, are estimated to have the metabolic syndrome (2). The metabolic syndrome refers to a constellation of interrelated cardiovascular risk factors that increase risk for the development of type 2 diabetes (T2DM) (3) and cardiovascular disease (4).
The morbidity and mortality associated with diabetes is profound. Diabetes is associated with multiple complications including heart disease, cerebrovascular disease, blindness, renal failure, neuropathy, lower extremity amputations, dental disease, and adverse pregnancy outcomes. Fortunately, complications can be reduced with glycemic control, cardiovascular risk reduction, and preventive care practices for eyes, kidneys, and feet. Unlike diabetes, for which there is widespread consensus regarding diagnostic criteria, metabolic syndrome is a poorly defined cluster of conditions. Furthermore, metabolic syndrome is not clearly associated with any risk beyond that attributable to its individual components. Because treatment currently involves nothing more than treating these individual components, the value of the diagnosis remains controversial.
The focus of this chapter will be on diabetes and hyperglycemia. We will begin by reviewing the various glucose tolerance categories. We will discuss the metabolic syndrome and its relationship to abnormal glucose tolerance and cardiovascular disease. Following a brief review of strategies to prevent diabetes, we will devote the remainder of the chapter to a discussion of therapeutic options for treating hyperglycemia. Other strategies to reduce cardiovascular disease (CVD) in diabetes, such as blood pressure control, treatment of hyperlipidemia, renin-angiotensin system blockade, antiplatelet therapy, and smoking cessation, are discussed in other chapters and will not be addressed here.
Glucose Tolerance Categories
Diabetes Mellitus
Diabetes mellitus refers to a spectrum of metabolic diseases characterized by hyperglycemia that results from defects in insulin secretion, insulin action, or both. The American Diabetes Association (ADA) recognizes four categories of diabetes with different underlying pathophysiologic mechanisms, as shown in Table 24-1. There is frequent overlap between the various types, and many patients do not easily fit into a single class. It is therefore less important to label the particular type of diabetes than it is to understand the pathogenesis of the hyperglycemia and to treat it effectively.
Prediabetes
The ADA Expert Committee recognizes an intermediate group of people whose glucose levels, although not meeting criteria
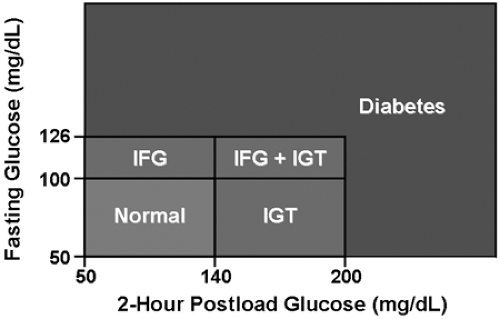
for diabetes, are too high to be considered normal (5) (Fig. 24-1). These individuals have been categorized as having impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT). IFG and IGT are often referred to as “prediabetes,” indicating the high risk for future development of T2DM. The annual rate of progression from prediabetes to T2DM is approximately 5% to 10% (6). Although this term may be useful in that it stresses the increased risk of diabetes associated with these conditions, it potentially excludes many people who are also at increased risk. Other important risk factors for diabetes include advanced age, excess adiposity, sedentary lifestyle, family history of diabetes, high-risk ethnic group, history of gestational diabetes, hypertension, dyslipidemia, polycystic ovarian syndrome, and history of vascular disease (7). Most individuals with prediabetes are euglycemic in their daily lives. They may experience transient elevation of the blood glucose during an acute illness, especially if amplified by certain drugs or intravenous glucose. This transient hyperglycemia, which likely indicates reduced insulin sensitivity or secretory capacity, is sometimes the first clue to incipient diabetes. Although the hyperglycemia may resolve immediately following resolution of the illness, it is important to recognize these patients as being at increased risk for T2DM. Regular screening for diabetes should be incorporated into their subsequent medical care.
for diabetes, are too high to be considered normal (5) (Fig. 24-1). These individuals have been categorized as having impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT). IFG and IGT are often referred to as “prediabetes,” indicating the high risk for future development of T2DM. The annual rate of progression from prediabetes to T2DM is approximately 5% to 10% (6). Although this term may be useful in that it stresses the increased risk of diabetes associated with these conditions, it potentially excludes many people who are also at increased risk. Other important risk factors for diabetes include advanced age, excess adiposity, sedentary lifestyle, family history of diabetes, high-risk ethnic group, history of gestational diabetes, hypertension, dyslipidemia, polycystic ovarian syndrome, and history of vascular disease (7). Most individuals with prediabetes are euglycemic in their daily lives. They may experience transient elevation of the blood glucose during an acute illness, especially if amplified by certain drugs or intravenous glucose. This transient hyperglycemia, which likely indicates reduced insulin sensitivity or secretory capacity, is sometimes the first clue to incipient diabetes. Although the hyperglycemia may resolve immediately following resolution of the illness, it is important to recognize these patients as being at increased risk for T2DM. Regular screening for diabetes should be incorporated into their subsequent medical care.
Table 24-1. Categories Of Diabetes Mellitus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Although there is increasing evidence that prediabetes increases risk for CVD and CVD mortality independent of traditional cardiovascular risk factors (8), there is no clear evidence that lowering glucose at this stage will reduce diabetes complications. On the other hand, because prediabetes is frequently associated with the metabolic syndrome (see following), components of the syndrome should be sought out and treated.
Metabolic Syndrome
The metabolic syndrome refers to a constellation of CVD risk factors that are associated with increased risk of diabetes and CVD (3,4,9). Over the past couple of decades, this cluster of clinical conditions has been referred to by several other names, including Syndrome X, the Dysmetabolic Syndrome, and the Insulin Resistance Syndrome. Key components of the syndrome include obesity, abnormal glucose metabo-lism, dyslipidemia, and hypertension. Insulin resistance and microalbuminuria are included in some, but not all, definitions. Although insulin resistance is widely considered to be the hallmark of the metabolic syndrome (9,10), it is not uniformly accepted to be the primary pathophysiologic abnormality (11). Multiple sets of diagnostic criteria have been described, each with slightly different components and diagnostic cut points, as shown in Table 24-2 (10,12,13,14,15).
The metabolic syndrome has been associated with increased cardiovascular risk in patients without diabetes (16,17,18) and with T2DM (18). It has also been associated with increased risk of diabetic nephropathy and poor glycemic control in type 1 diabetes (T1DM) (19). Although there is no question that certain CVD risk factors are prone to cluster together, recent studies have questioned whether the metabolic syndrome predicts CVD better than established risk assessment models (16,20) or than the sum of its parts (11).
A recent joint statement by the ADA and the European Association for the Study of Diabetes challenged the prognostic and therapeutic utility of the metabolic syndrome as it is currently conceived (11). Although most clinicians are now aware of the metabolic syndrome, there is little consensus about its definition and significance. Different definitions are ambiguous and discordant. For example, obesity is defined by body mass index (BMI) in some definitions and by waist circumference in others. Furthermore, the dichotomous diagnostic cut points may not account for the spectrum of risk that is likely attributable to varying degrees of abnormality. The value of diagnosing this syndrome also has been questioned because no specific therapies (beyond those established for its individual components) have been deemed effective (11).
Prevention of Diabetes
Prevention of T1DM
To date, trials aimed at preventing T1DM have not been successful. Treatments with parenteral insulin (21), oral insulin (22), and oral nicotinamide (23) have not been effective in preventing T1DM among relatives of patients with T1DM. Nevertheless, this remains an area of intense investigation. Multiple trials aimed to prevent T1DM or to delay the progressive loss of β-cell function in newly diagnosed patients are currently in progress.
Prevention of T2DM
It has been estimated that 20% to 60% of people with newly diagnosed T2DM already have a complication at the time of diagnosis (24,25). Consequently, there is intense interest in preventing diabetes, and many trials have studied whether the onset of T2DM can be avoided or delayed (6,26). The hope is that forestalling the development of T2DM would likewise reduce diabetic complications. However, the impact of diabetes prevention on diabetes complications is currently unknown. It is likely that diabetes prevention would reduce microvascular complications (retinopathy, nephropathy, and neuropathy), which are strongly associated with hyperglycemia (25). However, because people with prediabetes and metabolic syndrome have an increased risk for CVD even in the absence of overt hyperglycemia, preventing T2DM in these individuals may not significantly reduce cardiovascular risk.
The major diabetes prevention trials are outlined in Table 24-3. Lifestyle changes (27,28) and treatment with metformin (28), troglitazone (29,30), and acarbose (31) have all been shown to reduce rates of progression to T2DM among high-risk patients. It is still not known whether these interventions truly prevent diabetes or simply delay its inevitable diagnosis.
Post-trial testing after discontinuation of metformin (32) and troglitazone (30) suggest persistent reductions in progression to diabetes, but further studies will be needed to establish that these treatments significantly alter the natural history of disease.
Post-trial testing after discontinuation of metformin (32) and troglitazone (30) suggest persistent reductions in progression to diabetes, but further studies will be needed to establish that these treatments significantly alter the natural history of disease.
Table 24-2. Components Of The Metabolic Syndrome | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Table 24-3. Major T2DM Prevention Trials | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
At this point in time, lifestyle modification remains the preferred approach to diabetes prevention. Weight loss and increased physical activity have beneficial effects on the entire cardiovascular risk profile (33,34,35) in addition to potential benefits in delaying the onset of diabetes. Unfortunately, lifestyle modification is difficult to achieve and maintain. Pharmacologic therapy to prevent T2DM may become an important therapeutic modality when lifestyle interventions are not sufficiently potent or are not feasible. Currently, there is insufficient evidence that pharmacologic therapy can produce sustained effects. Furthermore, its safety and cost-effectiveness are unknown.
Prevention of Diabetes Complications
Because diabetes is considered a coronary heart disease risk equivalent (36), patients with diabetes should be treated with the same aggressive cardioprotective strategies that are recommended for nondiabetic patients with known CVD. These therapies are presented in detail in other chapters and will not be discussed here.
Glycemic control remains a central focus of treatment for diabetic patients. Hemoglobin A1c (A1C), a measure of long-term glycemic control, is used to guide therapy. Although elevated A1C levels are strongly associated with microvascular disease (25,37), the role of glycemic control in the development of CVD has been more controversial. Prospective studies have clearly demonstrated reductions in microvascular complications with intensive glycemic control in both T1DM (37) and T2DM (25). Although reductions in CVD were not found in these studies, emerging prospective (38,39) and epidemiologic data (40,41) suggest that glycemic control may also reduce CVD risk.
In the Diabetes Control and Complications Trial (DCCT), intensive glycemic control in T1DM (A1C 7.2% vs. 9.1%) reduced the risk of retinopathy by 76%, the risk of microalbuminuria by 34%, and the risk of neuropathy by 69% (37). Although CVD event rates were not reduced in the initial study, observational follow-up studies of DCCT participants 6 to 10 years after completion of the initial trial revealed significant reductions in carotid intima-media thickness (38) and fewer CVD events (39) among the participants who had originally been assigned to intensive therapy. These data suggest a delayed effect of glycemic control on cardiovascular risk.
In the United Kingdom Prospective Diabetes Study (UKPDS) of patients with T2DM, a 1% reduction in A1C was associated with a 25% reduction in microvascular complications in the intensively treated group (median A1C = 7.0%) compared with the conventionally treated group (median A1C = 7.9%) after 10 years of follow-up (P = 0.0099) (25). There was a 16% reduction (P = 0.052) in myocardial infarction observed for the intensively treated group compared with the conventionally treated group. Although this difference did not reach statistical significance, these findings suggest that glycemic control may protect against CVD in T2DM.
Recently there has been increasing evidence suggesting that increased postprandial glucose values also play a role in the development of CVD. Furthermore, reducing postprandial hyperglycemia may decrease atherosclerotic risk. Reduction of postprandial hyperglycemia has been associated with carotid intima-media thickness regression, reductions in inflammatory markers, and improved endothelial function (40,41,42).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree