Device Therapy in Heart Failure: Selection of Patients for ICD and CRT
Eli V. Gelfand
Peter J. Zimetbaum
Heart failure (HF) currently represents a major public health problem in the United States. Superior treatment strategies for acute coronary syndromes have created a large population of acute myocardial infarction (MI) survivors with poor left ventricular (LV) function. The growing burden of diabetes mellitus and undertreated hypertension contribute to a rising prevalence of HF. As a result, HF is now the leading hospital discharge diagnosis and accounts for 15 million office visits in the United States (1).
A clinical diagnosis of HF portends an increased risk of ventricular arrhythmias and sudden cardiac death (SCD). However, even in absence of congestive symptoms, a dilated, hypocontractile left ventricle is a marker of an increased risk. Whereas for patients with advanced (New York Heart Association [NYHA] class III-IV) congestive heart failure (CHF), declining ventricular pump function, and organ failure due to hypoperfusion may represent the common mode of death, for patients with NYHA class II CHF, incidence of death from arrhythmia may dominate that from pump failure. The goals of device therapy in HF are to (a) prevent SCD, (b) reduce symptoms of heart failure, (c) attenuate or reverse cardiac remodeling, and (d) allow for a more aggressive pharmacologic therapy, proven to reduce mortality (Table 21-1).
Since the publication of secondary prevention clinical trials conducted in the 1990s, there has been little controversy over the use of implantable devices to prevent recurrent cardiac arrest or hemodynamically unstable ventricular arrhythmias. The major focus of recent investigations has been on primary prevention of sudden cardiac death from arrhythmia in patients with HF.
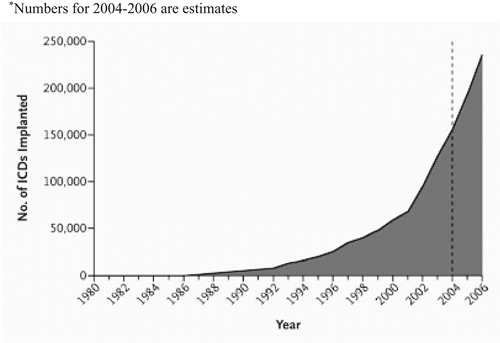
Concurrent with an ever-expanding body of data on device therapy, the device design itself has advanced at a rapid pace. In the 1970s, an implantable cardioverter defibrillator (ICD) was a relatively bulky device, which required a thoracotomy for implantation. Current-generation ICDs are microprocessor-driven units that weigh less than 60 g. They are implanted transvenously from a subcutaneous pocket and are capable not only of terminating a ventricular tachyarrhythmia by a direct-current countershock, but also of antitachycardia pacing, backup pacing for bradycardia, biventricular pacing, complex arrhythmia analysis, and electrogram storage. Improved battery design means that the life span of an average ICD has been extended to over 5 years. Driven largely by recent trial data, the number of device implants has skyrocketed in the United States in the late 1990s to the early 2000s (Fig. 21-1) (2).
The goal of this chapter is to suggest an evidence-based rationale for selection of patients with HF, for whom device therapy is indicated and appropriate. Also, it is our goal to aid the clinician with the selection of a proper device type for a particular patient population.
Types of Available Devices
ICD Components and Function
Implantable cardioverter defibrillators used in treatment of patients with HF are broadly divided into three types: single-chamber, dual-chamber, and biventricular (cardiac resynchronization therapy, CRT) ICDs. Most commercially available ICDs incorporate full pacemaker capabilities; however, detailed review of those features is outside the scope of this chapter. The basic components of any ICD system are similar (Fig. 21-2) and consist of a battery encased in a sealed titanium can, capacitors that store the energy prior to cardioversion/defibrillation, a microprocessor that analyzes the cardiac rhythm and governs the ICD function and a header, through which the leads are attached. The ICD leads function as both pacing electrodes, as well as defibrillation coils.
In a single-chamber defibrillator system (Fig. 21-2A), a single lead is implanted into the right ventricular apex or septum and thus is able to pace and defibrillate the ventricle. In a dual-chamber system (Fig. 21-2B), an atrial lead is added, which can track atrial activity and provide backup atrial pacing if necessary. A biventricular device (Fig. 21-2C) generally has three leads: an atrial pacing lead, a right ventricular pacing/defibrillation
lead, and a LV pacing lead. The LV lead is placed via the coronary sinus into one of the lateral cardiac veins overlying the LV surface. Biventricular devices are available with and without defibrillator capability.
lead, and a LV pacing lead. The LV lead is placed via the coronary sinus into one of the lateral cardiac veins overlying the LV surface. Biventricular devices are available with and without defibrillator capability.
Table 21-1. Rationale for Pursuing Device Therapy in Patients with Congestive Heart Failure with Depressed Left Ventricular Function | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
Device Implantation
Implantation of an ICD is typically performed under conscious sedation, in much the same way as a pacemaker insertion. Complications of implantation are uncommon, but include cardiac perforation with tamponade, venous trauma, bleeding into the device pocket, and lead dislodgment. Most important long-term complication of ICD therapy is device or lead infection. If device infection is diagnosed, prolonged antibiotic therapy is mandated, and device removal with lead explantation is often required. The latter is associated with an approximately 2.5% risk of serious complications, including central venous trauma, hemopericardium, and hemothorax (3).
Device Therapy in Secondary Prevention of Sudden Cardiac Death
It is now recognized that in absence of a reversible cause of sudden cardiac death (e.g., acute ischemia causing ventricular tachycardia [VT] or ventricular fibrillation [VF]), the risk of SCD recurrence is high. Three important randomized, controlled clinical trials have demonstrated that when compared
with antiarrhythmic therapy, ICD therapy is associated with a decrease in the incidence of recurrent SCD (Table 21-2) (4,5,6).
with antiarrhythmic therapy, ICD therapy is associated with a decrease in the incidence of recurrent SCD (Table 21-2) (4,5,6).
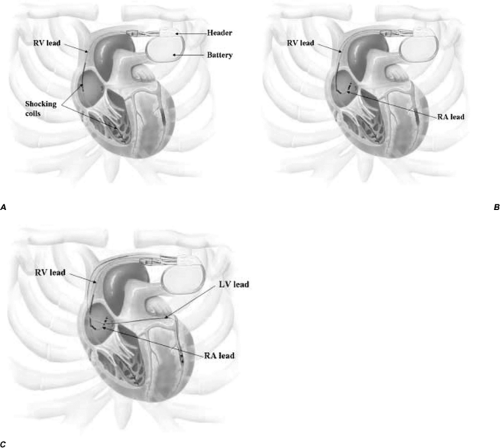 Figure 21-2. Basic components of commonly available heart failure devices. Images provided courtesy of Guidant Corp. (A) single-chamber ICD, (B) dual-chamber ICD, (C) biventricular ICD. |
In the Antiarrhythmic Versus Implantable Defibrillator (AVID) trial (4), mortality after 2 years was 18.4% in the ICD group, and 25.3% in the antiarrhythmic group, yielding an approximately 30% relative decrease in all-cause mortality, which persisted through 3 years of follow-up. Most of the patients in the AVID antiarrhythmic group received amiodarone. These results were confirmed in the Canadian Implantable Defibrillator (CIDS) trial (5), in which patients were randomly assigned to receive amiodarone or an ICD. A 20% relative risk reduction in all-cause mortality over 3 years of follow-up was observed, although it did not reach statistical significance. Critique of AVID and CIDS included the more frequent use ofβ-blockers in the ICD arms of both trials, which may have biased the findings toward device therapy. An 11-year follow-up from a single-center subset of CIDS demonstrated that the benefit of ICD therapy over amiodarone increases over time (7). Additionally, the majority of patients treated with amiodarone (82%) developed significant adverse effects from the medication, resulting in discontinuation in 50% of the patients. The third trial in the series was the Cardiac Arrest Study Hamburg (CASH) (6), in which patients were randomly assigned to receive a β-blocker metoprolol, amiodarone, or ICD. In this trial, ICD was again superior to antiarrhythmic therapy (both metoprolol and amiodarone), with an approximately 30% reduction in SCD mortality in the device group.
Meta-analysis of AVID, CIDS, and CASH (8) was published in 2005 and demonstrated that among survivors of SCD, defibrillator therapy was associated with a 28% relative risk reduction of all-cause mortality, which was almost entirely to a 50% reduction in the risk of recurrent SCD. Over the follow-up period of 6 years, ICD therapy prolonged patient survival by 4.4 months compared to antiarrhythmic therapy.
Based on the findings of these randomized trials, ICD therapy is a Class I recommendation (beneficial, useful, and effective treatment) for patients who suffered cardiac arrest from VT or VF, or had unstable/high risk VT (Table 21-3).
Table 21-2. Selected Major Clinical Trials of Device Therapy for Secondary Prevention of Sudden Cardiac Death | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Device Therapy in Primary Prevention of Sudden Cardiac Death
Survival from an out-of-hospital cardiac arrest remains low despite a significant investment in such effective public health measures as automated external defibrillators (AEDs) (9). Therefore, identification of patients at high risk for a first episode of SCD has been and remains an important scientific and public health priority. In an attempt to define such a population, randomized clinical trials enrolled progressively broader groups of patients and tested ICD therapy as means of primary prevention of SCD (Table 21-4).
Coronary disease in combination with poor ventricular function is thought to portend an especially high risk of malignant ventricular arrhythmias. The first trial to demonstrate benefit of ICD over antiarrhythmic therapy was the small Multicenter Automatic Defibrillator Implantation Trial (MADIT) (10), which enrolled patients with a documented history of MI, ejection fraction (EF) ≤35%, and nonsustained ventricular tachycardia (NSVT). Patients underwent electrophysiological (EP) study, and if VT could be induced but not suppressed, they were randomized to antiarrhythmic therapy or ICD. Over the course of follow-up, patients in the ICD group benefitted from a 54% reduction in mortality compared to placebo. Based on the results of MADIT (and despite the small sample size of the study, as well as the more frequent use of β-blockers in the ICD group), ICD therapy was approved for this indication. A much larger Multicenter Unsustained Tachycardia Trial (MUSTT) (11) was a study comparing a medical approach with an electrophysiology-guided approach for primary prevention of SCD. Patients were enrolled if they had a history of MI, EF ≤40%, and NSVT. Though MUSTT was not designed as a “device trial,” its protocol specified that a subset of patients in the EP-guided group have an ICD implanted. Comparison of this subpopulation with matched antiarrhythmic controls demonstrated a marked benefit to ICD therapy (a 76% decrease in the incidence of SCD), indirectly supporting the results of MADIT. In comparison with MADIT-I and MUSTT, the second MADIT trial (MADIT-II) markedly widened its enrollment criteria (12). It enrolled patients with a history of MI and EF ≤30%. No documentation of NSVT, or proof of VT inducibility in the EP lab was required. Over 1,200 patients were randomized to ICD or conventional therapy, and 31% mortality reduction with ICD therapy compared to control was observed beginning at 9 months after implantation (14.2% vs. 19.8%, P = 0.016). The study was stopped prematurely because of the marked benefit in the ICD group.
Table 21-3. Recommendations for ICD Therapy in Secondary Prevention of Sudden Cardiac Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
How early after the first determination of poor LV function is made should the ICD be implanted? Indeed, do patients with myocardial stunning and low LV EF early after MI represent appropriate candidates for ICD therapy? The DINAMIT trial (13) answered this question in a population of 674 patients with a recent (<40 days) MI and EF ≤35%. Desire to identify an even higher-risk group among this population led to randomization
only of those patients who demonstrated abnormal heart rate variability—a marker of autonomic dysfunction. ICD therapy in this group, when compared to conventional treatment, demonstrated no benefit in reducing mortality after 2.5 years of follow-up.
only of those patients who demonstrated abnormal heart rate variability—a marker of autonomic dysfunction. ICD therapy in this group, when compared to conventional treatment, demonstrated no benefit in reducing mortality after 2.5 years of follow-up.
Table 21-4. Elected Major Clinical Trials of Device Therapy for Primary Prevention of Sudden Cardiac Death | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree