The aim of the present study was to evaluate whether diagnostic data collected after a heart failure (HF) hospitalization can identify patients with HF at risk of early readmission. The diagnostic data from cardiac resynchronization therapy defibrillator (CRT-D) devices can identify outpatient HF patients at risk of future HF events. In the present retrospective analysis of 4 studies, we identified patients with CRT-D devices, with a HF admission, and 30-day postdischarge follow-up data. The evaluation of the diagnostic data for impedance, atrial fibrillation, ventricular heart rate during atrial fibrillation, loss of CRT-D pacing, night heart rate, and heart rate variability was modeled to simulate a review of the first 7 days after discharge on the seventh day. Using a combined score created from the device parameters that were significant univariate predictors of 30-day HF readmission, 3 risk groups were created. A Cox proportional hazards model adjusting for age, gender, New York Heart Association class, and length of stay during the index hospitalization was used to compare the groups. The study cohort of 166 patients experienced a total of 254 HF hospitalizations, with 34 readmissions within 30 days. Daily impedance, high atrial fibrillation burden with poor rate control (>90 beat/min) or reduced CRT-D pacing (<90% pacing), and night heart rate >80 beats/min were significant univariate predictors of 30-day HF readmission. Patients in the “high”-risk group for the combined diagnostic had a significantly greater risk (hazard ratio 25.4, 95% confidence interval 3.6 to 179.0, p = 0.001) compared to the “low”-risk group for 30-day readmission for HF. In conclusion, device-derived HF diagnostic criteria evaluated 7 days after discharge identified patients at significantly greater risk of a HF event within 30 days after discharge.
The ability of device diagnostics to risk stratify patients with heart failure (HF) during the vulnerable postdischarge period after a HF admission has not been addressed by earlier studies. Using the paradigm of improving outcomes through early interactions with patients with HF, the present analysis was undertaken to assess the ability of device diagnostic information during the 7 days after discharge to identify patients at risk of readmission within 30 days of an index HF admission.
Methods
The present retrospective analysis included data available from the Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure (PARTNERS-HF) (n = 694 patients), The OptiVol Fluid-Index InSync Sentry Registry (OFISSER) (n = 323 patients), Fluid Accumulation Status Trial (FAST) (n = 146 patients), Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision (CONNECT) (n = 313 patients) studies, and case study files (n = 80 patients). For the CONNECT study, only the control arm patients with cardiac resynchronization therapy defibrillator (CRT-D) devices, who were not being monitored because of atrial fibrillation (AF) diagnostic alerts, were included in the analysis. Only patients with CRT-D devices in these studies with ≥90 days of follow-up device data were included for a final initial cohort of 1,561 patients. From this initial cohort, the patients were included in the present analysis if they had a HF hospitalization and 30 days of follow-up data after discharge. Each cardiovascular hospitalization was adjudicated for signs and symptoms of HF that included administration of intravenous or oral diuretics during the hospitalization.
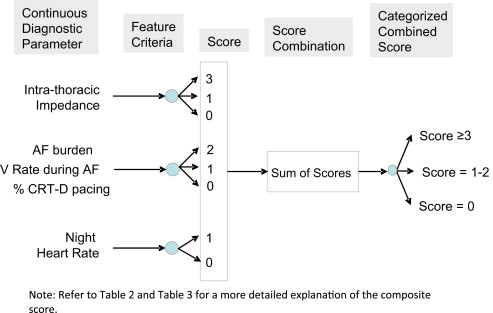
The device diagnostics evaluated in the present study are specific to Medtronic (Mounds View, Minnesota) CRT-D devices. The 4 diagnostic parameters investigated in the present study were intrathoracic impedance, AF diagnostics, night heart rate (NHR), and heart rate variability (HRV). Intrathoracic impedance studies the cumulative difference between the reference impedance and the daily impedance. The daily intrathoracic impedance is calculated from the voltage measured from an asynchronous current applied between the right ventricular lead coil electrode and the device case electrode. The reference impedance is initialized on day 34 after implantation as the average of the last 4 days of daily impedance measurement. The reference impedance increases or decreases by a fixed amount depending on whether a 4-day weighted average of daily impedance is greater than or less than the reference impedance. The AF burden, the ventricular rate during AF, and the percentage of CRT-D pacing were combined into the AF plus rapid ventricular response diagnostics because they correlated with each other. The AF burden includes atrial fibrillation, atrial tachycardia, and atrial flutter. The NHR is the average ventricular rate from 12 a.m. (midnight) to 4 a.m. The HRV is computed each day using the median atrial heart rate, determined every 5 minutes. The HRV is not computed on any day the atrial pacing was >80% of the time or if the patient was in AF.
For each HF hospitalization, the HF diagnostics information was evaluated in the 7 days after discharge to investigate whether a combined diagnostic criterion can identify the patients who are at a greater risk of readmission for HF within the 30-day period after discharge after an index hospitalization for HF. For patients with multiple HF hospitalizations, each hospitalization was considered as an index hospitalization for the purpose of the analysis if 30 days of follow-up information after discharge were available from that hospitalization. The modeling of a 7-day review of device data covering the first 7 days after discharge is based on previous research that suggests that clinic visits within 7 days of discharge can reduce all-cause 30-day readmission.
Each of the diagnostic parameters was analyzed in a univariate fashion to determine the parameter’s ability to identify patients at risk of HF hospitalization using the Anderson-Gill model, an extension of the Cox proportional hazards model that accounts for multiple index HF hospitalizations in patients. Thresholds were identified for each parameter to identify different categories of risk. The thresholds were chosen from a large number of investigated possibilities such that they provided the best ability for the diagnostic parameter to identify patients at risk of a 30-day readmission for HF. The goal was to identify a threshold for high risk that was triggered 5 to 10% of the time for the cohort of patients discharged alive from the index HF hospitalization. Using the hazard ratio (HR) identified by a multivariate model that included all the diagnostic parameters, the scores were assigned empirically to each level of risk and for missing values to provide weighting for predicting HF readmission. If diagnostic data were missing (i.e., data were invalid or not applicable), a score of 0 was assigned to indicate that no information was available from the diagnostic parameter. If the diagnostic parameter did not reach statistical significance as a univariate predictor, no scores (equivalent to a score of 0) were assigned for that diagnostic parameter. Empirical weights and whole number threshold values were preferred such that the risk stratification method can be easily implemented in clinical practice using a simple and least burdensome evaluation of the device diagnostics reports available today.
A combined score was tabulated by adding the scores created for each of the diagnostic parameters ( Figure 1 ). The combined score was then used to identify 3 levels of risk (low, medium, and high) for HF readmission among discharged patients with HF. The thresholds for the different categories were chosen such that the high-risk cohort had approximately the top 20% of the combined scores and the low-risk cohort had the bottom 20% of the combined scores. The medium risk cohort included the composite scores not classified as “high” or “low.” The Anderson-Gill model, with adjustment for multiple index HF hospitalizations in patients, was used to determine the ability of the combined score risk groups to identify patients at risk of 30-day readmission for HF. Because the diagnostic assessment was performed during the first 7 days after discharge (thus creating a possibility of biased association), the analysis was also performed after excluding index HF hospitalizations with readmission within the first 7 days after discharge. To evaluate whether considering multiple index hospitalizations in the same patient significantly biased the results, the assessment was also performed considering the first HF hospitalization as the only index hospitalization in each patient.
Results
For the 1,561 patients identified from the 4 studies, the average follow-up duration was 373 ± 146 days. A total of 331 hospitalizations were adjudicated as HF related in 208 patients (13%), with an event rate of 0.2/patient-year of monitoring. After censoring hospitalizations without ≥30 days of follow-up information after discharge, the HF hospitalization group included 254 HF hospitalizations in 166 patients. Postdischarge readmission for HF occurred within 30 days for 27 patients (16% of admitted patients). This cohort of admitted patients experienced a total of 34 readmissions with HF as the primary cause. Although notable differences were seen in the baseline characteristics, including a 19% increase in diabetes in those who were readmitted, none of these differences reached statistical significance in this small group of patients ( Table 1 ).
| 30-d Rehospitalization | ||||
|---|---|---|---|---|
| Variable | Total (n = 166) | Yes (n = 27) | No (n = 139) | p Value |
| Mean age (y) | 70 ± 10 | 69 (9) | 70 (10) | 0.52 |
| Men | 73% | 78% | 72% | 0.55 |
| Mean ejection fraction | 23 ± 9% | 23 ± 8% | 23 ± 9% | 1.00 |
| Ejection fraction ≤35% | 99% | 100% | 98% | 0.55 |
| New York Heart Association classification | 0.75 | |||
| I | 2% | 4% | 2% | |
| II | 8% | 7% | 8% | |
| III | 78% | 81% | 77% | |
| IV | 12% | 7% | 13% | |
| Coronary artery disease | 79% | 81% | 78% | 0.70 |
| Hypertension | 79% | 68% | 81% | 0.18 |
| Diabetes mellitus | 48% | 64% | 45% | 0.12 |
| Myocardial infarction | 48% | 59% | 46% | 0.21 |
| Atrial fibrillation | 41% | 41% | 41% | 1.00 |
| Baseline medications | ||||
| Angiotensin-converting enzyme/angiotensin receptor blocker | 68% | 63% | 69% | 0.51 |
| β Blockers | 86% | 81% | 87% | 0.46 |
| Diuretics | 91% | 93% | 91% | 0.82 |
| Digoxin | 34% | 37% | 34% | 0.73 |
| Aldosterone receptor blocker | 33% | 26% | 34% | 0.40 |
| Antiarrhythmic drug | 32% | 22% | 34% | 0.22 |
| Anticoagulation | 82% | 81% | 82% | 0.90 |
| Warfarin | 41% | 37% | 42% | 0.66 |
The device diagnostic parameter thresholds predictive of 30-day HF readmission were identified and categorized as high, intermediate, and low ( Table 2 ). The total number of index hospitalizations (i.e., sample size) in each parameter risk category and the percentage of those followed by 30-day readmission for HF (i.e., event rate) are listed in Table 3 . Impedance, AF, AF plus rapid ventricular response, and NHR all had the ability to identify risk for 30-day readmission as a univariate predictor. In the multivariate model with the diagnostics only, impedance and AF plus rapid ventricular response were independent predictors of 30-day readmission. According to the strength of the diagnostic parameter to predict readmission, an empirical weighting for each parameter was created. The strongest predictor of 30-day HF readmission with a weighted score of 3 was daily impedance that stayed significantly and consistently below the reference impedance during the 7 days after discharge. As noted in the Table 3 , each significant univariate predictor at identified thresholds was assigned a score.
| Diagnostic Parameter | HF Hospitalizations (n) | 30-day HF Readmission (n) | HR (95% CI) | p Value | Score |
|---|---|---|---|---|---|
| Impedance | |||||
| High | 27 (11%) | 9 (33.3%) | 8.23 (2.5–26.7) | <0.001 | 3 |
| Intermediate | 129 (53%) | 18 (14.0%) | 3.19 (1.1–9.0) | 0.028 | 1 |
| Low | 88 (36%) | 4 (4.5%) | Reference | 0 | |
| Atrial fibrillation and rapid ventricular rate | |||||
| High | 14 (6%) | 6 (42.9%) | 6.50 (2.7–15.4) | <0.001 | 2 |
| Intermediate | 45 (18%) | 11 (24.4%) | 3.03 (1.4–6.4) | 0.004 | 1 |
| Low | 195 (77%) | 17 (8.7%) | Reference | 0 | |
| Night heart rate | |||||
| Intermediate | 98 (41%) | 20 (20.4%) | 2.51 (1.2–5.1) | 0.011 | 1 |
| Low | 139 (59%) | 12 (8.6%) | Reference | 0 | |
Scores were not assigned for HRV criteria, because the diagnostic parameter was not found to be a statistically significant univariate predictor. Because of atrial pacing or atrial tachycardia/AF, HRV data were missing during the 7 days after discharge for 86 of the 254 index hospitalizations. Of the 34 30-day readmissions for HF, 12 (35%) occurred when the postindex hospitalization HRV data were missing.
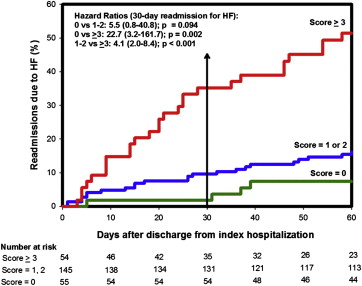
The combined score obtained by adding the scores for each individual parameter risk category with appropriate weighting was divided into 3 risk groups. The Kaplan-Meier curve for incidence of readmission for HF after discharge from the index hospitalization for the 3 groups is shown in Figure 2 . Of the patients in the high-risk group (score ≥3; n = 54), 35% and 51% were readmitted for HF within 30 and 60 days, respectively. In contrast, only 2% and 7% of the patients in the low-risk group (score = 0; n = 55) were readmitted for HF within 30 and 60 days, respectively. The high-risk cohort was 23 times more likely to be readmitted within 30 days for HF compared to the low-risk cohort (HR 22.7, 95% confidence interval [CI] 3.2 to 161.7, p = 0.002). Patients with HF with a combined score in the medium-risk category (score 1 to 2) were at a nonsignificant increased risk of HF readmission (14 readmissions, 9.7%) compare to those with a low-risk combined score (HR 5.5, 95% CI 0.8 to 40.8). The high-risk cohort was also at a significantly increased risk of readmission compared to the medium-risk group (HR 4.11, 95% CI 2.01 to 8.39, p <0.001). The sensitivity, specificity, and positive and negative predictive values for a combined risk score of ≥3 was 56%, 84%, 35%, and 93%, respectively. For patients with a combined score of ≥1 (intermediate- and high-risk groups), the sensitivity, specificity, and positive and negative predictive value was 97%, 25%, 17%, and 98%, respectively.