Previous models for contrast-induced acute kidney injury (CI-AKI) after percutaneous coronary intervention (PCI) include procedure-related variables in addition to pre-procedural variables. We sought to develop a risk model for CI-AKI based on pre-procedural variables and compare its predictability with a conventional risk model and also to develop an integer score system based on selected variables. A total of 5,936 consecutive PCIs registered in the Japanese Cardiovascular Database were analyzed (derivation cohort, n = 3,957; validation cohort, n = 1,979). CI-AKI was defined as an increase in serum creatinine of 50% or 0.3 mg/dl compared with baseline. From the derivation cohort, 2 different CI-AKI risk models were generated using logistic regression analyses: a pre-procedural model and a conventional model including both pre-procedural and procedure-related variables. The predictabilities of the models were compared by c-statistics. An integer score was assigned to each variable in proportion to each estimated regression coefficient for the final model. In our derivation cohort, the proportion of CI-AKI was 9.0% (n = 358). Predictors for CI-AKI included older age, heart failure, diabetes, previous PCI, hypertension, higher baseline creatinine level, and acute coronary syndrome. Presence of procedure-related complications and insertion of intra-aortic balloon pumping were included as procedure-related variables in the conventional model. Both the conventional model (c-statistics 0.789) and the pre-procedural model (c-statistics 0.799) demonstrated reasonable discrimination. The integer risk-scoring method demonstrated good agreement between the expected and observed risks of CI-AKI in the validation cohort. In conclusion, the pre-procedural risk model for CI-AKI had acceptable discrimination compared with the conventional model and may aid in risk stratification of CI-AKI before PCI.
Contrast-induced acute kidney injury (CI-AKI) is a common complication of percutaneous coronary intervention (PCI) and is associated with increased risk of morbidity and short- and long-term mortality. Various risk score models have been proposed to identify the patients at risk of CI-AKI as the therapeutic options are limited and a prophylactic approach is crucial for this entity. However, the previously established risk scores have not been fully exploited in current clinical practice because they include not only pre-procedural but also procedure-related variables, which make it difficult to pre-procedurally identify the patients at risk of CI-AKI. Thus, to improve the pre-procedural stratification of patients at risk of CI-AKI, the development of a risk model without procedure-related variables is of utmost importance. Here, we sought to develop 2 different risk models, one based on pre-procedural variables only and the other based on all available variables, including both pre-procedure– and procedure-related variables, using data from a Japanese Multicenter PCI Registry, and to compare their predictive abilities. By demonstrating the sufficient predictive ability of a pre-procedural risk model of CI-AKI, pre-procedural stratification of patients at risk can be improved.
Methods
Data for the development and validation of CI-AKI risk models were derived from the Japan Cardiovascular Database Keio Inter-hospital Cardiovascular Studies (JCD-KICS), which is a prospective multicenter registry designed to collect clinical variables and outcome data on consecutive patients with PCI, with dedicated clinical research co-ordinators assigned to each site. Approximately 200 variables are collected for each patient. The clinical variables and in-hospital outcomes for JCD-KICS were defined in accordance with NCDR, version 4.1. This registry, sponsored by the American College of Cardiology, is the largest national clinical registry program for diagnostic cardiac catheterization and PCI, with >1,500 centers currently participating across the United States. The JCD-KICS includes 16 teaching hospitals within the metropolitan Tokyo area, and the participating hospitals were instructed to record and register data from consecutive hospital visits for PCI using an Internet-based database system. All PCI procedures performed with any commercially available coronary device were included. The data entered were checked for completeness and internal consistency. Quality assurance of the data was achieved through automatic system validation and reporting of data completeness and through education and training for dedicated clinical research co-ordinators specifically trained for the present PCI registry. The senior study co-ordinator (IU) and exclusive on-site auditing by the investigators (SK and AK) ensured proper registration of each patient.
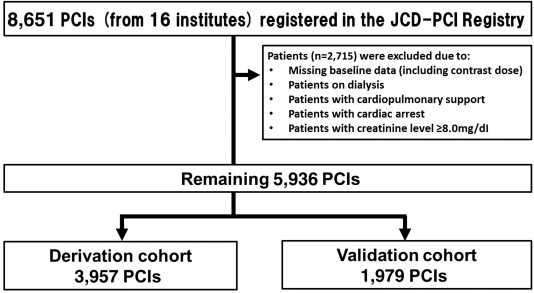
A total of 8,651 patients who underwent PCI procedures from January 2011 to March 2013 for acute and nonacute indications were registered in the database. Although JCD-KICS have collected data from September 2008, we excluded data from September 2008 to December 2010 in this analysis because information regarding dosing of contract media has only been collected since January 2011. A total of 2,715 patients were excluded because they were on dialysis or cardiopulmonary support, had cardiac arrest, and had serious renal dysfunction (serum creatinine ≥8.0 mg/dl), or because of insufficient baseline data, resulting in 5,936 patients being included in our study ( Figure 1 ).
An interventional team performed PCIs according to the standard clinical practice through the femoral or radial approach. Supportive pharmacologic therapies, mechanical support, contrast medium dose (nonionic low osmolar), and the angioplasty technique were left to the discretion of the operators, according to each institution’s clinical protocols and the international guidelines. Whether to perform pre-procedural hydration and administer bicarbonate or N-acetylcysteine was also left to the operators. Cessation of the use of nephrotoxic medications, such as biguanide or nonsteroidal anti-inflammatory drugs, was encouraged before admission in elective cases and after admission in emergent cases.
CI-AKI was defined as increase in serum creatinine of 50% or 0.3 mg/dl after PCI compared with the baseline value. Postprocedural creatinine value was defined as the highest value within 30 days after indexed procedure based on the definition of NCDR CathPCI registry ; therefore, if >1 postprocedural creatinine was measured, the highest value was used for CI-AKI calculation. Anemia was defined using the World Health Organization criteria as baseline hemoglobin value <13 g/dl for men and <12 g/dl for women. Procedural complications included significant dissection, perforation, procedure-related myocardial infarction, cardiogenic shock, heart failure, ischemic or hemorrhagic stroke, tamponade, vascular complications requiring treatment, and bleeding. Bleeding was defined as follows: (1) occurring at the percutaneous entry site, during or after the catheterization laboratory visit until discharge, which may be external or a hematoma >10 cm for femoral, >5 cm for brachial, or >2 cm for radial access; (2) retroperitoneal; (3) gastrointestinal; (4) genitourinary; and (5) other/unknown origin during or after the catheterization laboratory visit until discharge. Only bleeding events requiring a transfusion and/or with a decrease in hemoglobin >3.0 g/dl were included. This bleeding criterion is also consistent with Bleeding Academic Research Consortium grade 3A to C. The definition of these complications was in accordance with the NCDR CathPCI registry, and any additional data elements and definitions can be found at their Web site.
The study cohort was randomly divided in a 2:1 ratio into derivation (n = 3,957) and validation (n = 1,979) cohorts, respectively. The demographic and clinical patient characteristics were summarized, and the data are presented as mean ± SD or as proportion (%), depending on the variables. In this study, we developed 2 different risk models: one based on pre-procedural variables only and one based on all available variables, including both pre- and procedure-related variables, from the derivation cohort, and compared their performance. Subsequently, we evaluated the validities of the developed risk models using the validation cohort.
A 2-step approach was used to identify the independent predictors of CI-AKI. First, from the derivation cohort, univariate analysis was performed to select significant risk factors of CI-AKI. Second, the set of identified predictors (p <0.10) was used as a pool of variables in constructing a final model using a backward stepwise multivariate logistic regression model, and the regression coefficients were estimated. In this model, age and body mass index (BMI) were treated as continuous covariates, and serum creatinine level (>1.0 mg/dl) and contrast dose (per 100 ml) were treated as categorical variables to make the model more clinically meaningful. We repeated the earlier mentioned method for the 2 different risk models, and c-statistics were used to compare the predictabilities of the 2 risk models. An integer score was assigned to each variable selected in the final model in proportion to the estimated regression coefficient defined from an incremental risk ratio per unit from the referencing age (50 years). This unit risk increment from the referencing age (0.024 for the pre-procedural risk model and 0.019 for the conventional risk model) was multiplied by 10, and the regression coefficient for each level of every risk factor was subsequently divided by this value (0.24 for pre-procedural risk model and 0.19 for conventional risk model) to compute its weights for the risk score.
Using the validation cohort data, the validities of the risk models with the integer scoring were also evaluated by examining the agreement between the predicted and observed proportions of CI-AKI in 5 groups defined with quintiles of the point totals. All data were analyzed using SPSS, version 21 (SPSS Inc., Chicago, Illinois), and the 2-sided significance level ( α ) was 0.05 for all analyses.
Results
Of the 3,957 patients in the derivation cohort, 358 patients (9.0%) experienced CI-AKI after PCI. Table 1 shows the baseline characteristics of the patients. The patients with CI-AKI tended to be older and women and to have lower BMI, significant heart failure status (New York Heart Association 3 or 4 equivalents), higher baseline creatinine level, and high proportion of co-morbidities, whereas patients without CI-AKI tended to have a higher prevalence of dyslipidemia and histories of myocardial infarction and PCI. There were also procedural differences between the patients with and without CI-AKI. Cardiogenic shock with or without intra-aortic balloon pump (IABP) support, emergent cases such as PCI for acute coronary syndrome, and procedural complications were more frequently observed in the patients with CI-AKI. Meanwhile, the patients without CI-AKI tended to undergo PCI through radial approach. Furthermore, in patients with CI-AKI, a higher amount of contrast media was used.
| Variable | Contrast-induced acute kidney injury | P value | |
|---|---|---|---|
| No | Yes | ||
| (N=3599) | (N=358) | ||
| Mean Age (Years) | 67.8±11.0 | 72.1±12.1 | <0.001 |
| Age ≥75 | 919 (25.5%) | 167 (46.6%) | <0.001 |
| Men | 2866 (79.6%) | 270 (75.4%) | 0.065 |
| Body mass index (kg/m 2 ) | 24.2±3.6 | 23.7±4.0 | 0.005 |
| <18.5 | 145 (4.1%) | 33 (9.4%) | <0.001 |
| New York Heart Association 3 or 4 | 200 (5.6%) | 66 (18.6%) | <0.001 |
| Diabetes Mellitus | 1424 (39.6%) | 168 (47.1%) | 0.007 |
| Previous Myocardial Infarction | 855 (23.8%) | 58 (16.2%) | 0.001 |
| Previous Percutaneous coronary Intervention | 1308 (36.3%) | 63 (17.6%) | <0.001 |
| Previous Coronary Artery Bypass Grafting | 186 (5.2%) | 15 (4.2%) | 0.527 |
| Cerebrovascular Disease | 301 (8.4%) | 51 (14.2%) | <0.001 |
| Peripheral Vascular Disease | 306 (8.5%) | 35 (9.8%) | 0.429 |
| Chronic Lung Disease | 115 (3.2%) | 13 (3.6%) | 0.637 |
| Hypertension | 2688 (74.7%) | 299 (83.5%) | <0.001 |
| Current/Recent Smoker | 1256 (34.9%) | 130 (36.5%) | 0.561 |
| Dyslipidemia | 2417 (67.2%) | 215 (60.1%) | 0.008 |
| Atrial Fibrillation | 139 (7.0%) | 18 (9.2%) | 0.247 |
| Anemia | 787 (28.0%) | 134 (41.1%) | <0.001 |
| Urgent or Emergent Procedure | 1634 (45.4%) | 269 (75.1%) | <0.001 |
| Acute Coronary Syndrome | 1743 (48.5%) | 273 (76.3%) | <0.001 |
| Radial approach | 1373 (38.1%) | 87 (24.3%) | <0.001 |
| Chronic total Occlusion | 106 (2.9%) | 4 (1.1%) | 0.042 |
| Multivessel Percutaneous Coronary Intervention | 312 (8.7%) | 44 (12.3%) | 0.019 |
| Periprocedural Complication | 226 (6.3%) | 93 (26.0%) | <0.001 |
| Periprocedural Bleeding | 80 (2.2%) | 9 (2.5%) | 0.415 |
| Cardiogenic shock | 61 (1.7%) | 22 (6.1%) | <0.001 |
| Intra-aortic Balloon Pump Support | 159 (4.4%) | 76 (21.2%) | <0.001 |
| Contrast dose (ml) | 178±79 | 187±88 | 0.027 |
| Creatinine | 0.93±0.41 | 1.15±0.67 | <0.001 |
| Creatinine >1.0mg/dL | 799 (22.2%) | 157 (43.9%) | <0.001 |
Table 2 shows the results of the multivariate logistic regression analyses. The clinical variables selected in the final model were older age, heart failure status, diabetes mellitus, no previous PCI, hypertension, higher baseline creatinine level (>1.0 mg/dl), and acute coronary syndrome. As procedure-related variables, presence of procedure-related complications and insertion of IABP were identified and included in the conventional model. Age was the only factor used as a continuous variable (>50 years), whereas all other factors were represented as categorical variables. Figure 2 demonstrates the receiver-operating characteristic curves. The c-statistics of pre-procedural and conventional models were 0.799 (95% confidence interval [CI] 0.783 to 0.815) and 0.789 (95% CI 0.773 to 0.805), respectively.
| Pre-procedural risk model | Conventional risk model | ||||
|---|---|---|---|---|---|
| β | OR (95% CI) | β | Odds Ratio | ||
| Number of years > 50 | 0.024 | 1.02 (1.01-1.04) | Number of years > 50 | 0.019 | 1.02 (1.01-1.03) |
| New York Heart Association 3 or 4 | 0.725 | 2.10 (1.46-2.92) | New York Heart Association 3 or 4 | 0.533 | 1.70 (1.18-2.46) |
| Diabetes Mellitus | 0.39 | 1.48 (1.15-1.90) | Diabetes Mellitus | 0.335 | 1.40 (1.08-1.81) |
| Previous Percutaneous Coronary Intervention | -0.751 | 0.47 (0.34-0.66) | Previous Percutaneous Coronary Intervention | -0.695 | 0.50 (0.35-0.71) |
| Hypertension | 0.383 | 1.47 (1.06-2.02) | Hypertension | 0.35 | 1.42 (1.02-1.97) |
| Pre-creatinine >1.0mg/dL | 0.845 | 2.33 (1.80-3.02) | Pre-creatinine >1.0mg/dL | 0.796 | 2.22 (1.70-2.89) |
| Acute Coronary Syndrome | 1.129 | 3.09 (2.25-4.25) | Acute Coronary Syndrome | 0.986 | 2.68 (1.94-3.71) |
| Not Applicable | Procedural Complication | 1.195 | 3.30 (2.37-4.60) | ||
| Intra-aortic Balloon Pump Insertion | 0.895 | 2.45 (1.70-3.52) | |||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


