Fig. 17.1
Two approaches to initiation of therapy in PAH: sequential combination therapy or up-front combination therapy
A.
Sequential Combination Therapy. Treatment is initiated with monotherapy and the outcome assessed. If treatment goals are not achieved, then additional, concomitant therapies are added one at a time.
B.
Up–Front Combination Therapy. Treatment is initiated with multiple medications simultaneously.
This chapter outlines the argument for the use of sequential addition of combination therapy as opposed to up-front combination therapy.
Biologic and Practical Rationale of Sequential Combination Therapy
The application of up-front combination therapy in PAH has drawn analogies to other diseases for which this strategy is commonly used, such as malignancy and Human Immunodeficiency Virus (HIV) infection [1, 2]. However, the molecular basis for this analogy is unclear. Monotherapy treatment for malignancy is associated with increased risk of de novo somatic mutations giving rise to resistant cellular clones; combination chemotherapy up-front decreases the chance of resistance, and prolongs survival in many forms of cancer [3]. Similarly, HIV may develop resistance to anti-retroviral therapy, which is prevented by the use of combination therapy [4]. However, there is no evidence that aberrant pulmonary vascular cells in patients with PAH develop escape mechanisms that are, in turn, resistant to therapy, much less that such patterns of resistance may be altered by up-front combination therapy.
Alternatively, the sequential addition of therapy allows evaluation of the individual clinical response to each medication, including allowing titration of the dose to optimize the benefit versus side effect profile. If the clinical response is (a) harm or (b) inadequate with the first medication, the initial medication can be removed or substituted, and/or a second class of medication added, with further optimization of the second medication before additional advancement of therapy as required. In this manner, tolerability and benefit are systematically optimized for each patient, with a clear assessment of response to each medication.
Such personalized therapy is important, as a fraction of patients will have a suboptimal response to any single medication. This fact of biologic variability is demonstrated by the data in Fig. 17.2, which reports the change in 6 min walk distance (6MWD) reported for PAH patients treated with riociguat in the recently published PATENT-1 trial [6]. In this study, 76 % of patients had a net positive improvement in 6MWD from baseline to the last study visit at 12 weeks, while 24 % of patients had a decline in 6MWD. The heterogeneity in clinical response will be compounded with the use of multiple medications, particularly if multiple medications are started simultaneously, some of which may be beneficial and some of which may be harmful; the net result may be partial or suboptimal, with great difficulty determining if one or more medications may actually be causing harm. Thus, sequential combination therapy has a strong biologic and practical rationale.
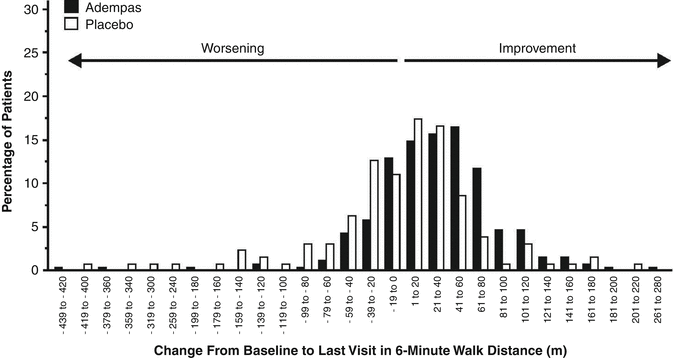

Fig. 17.2
Distribution of change in 6MWD for patients with PAH receiving riociguat in the PATENT-1 study (From the ADEMPAS monograph [5]; reproduced with permission of Bayer). Of the patients who received riociguat, 76 % experienced an improvement in 6MWD, and 24 % experienced a decrease in 6MWD
Clinical Evidence in Support of Sequential Combination Therapy
To date, 14 double-blind randomized clinical trials investigating the use of combination therapy in PAH have been published. Thirteen of these studies investigated the use of medications added sequentially (Table 17.1). In these studies, sequential addition of therapy was typically performed in the context of background PAH-specific therapy, with the exception of Iversen and colleagues, who assessed the effect of non-selective endothelin receptor antagonist therapy initiation with bosentan, followed by randomization of patients to placebo or sildenafil in a cross-over design [10].
Table 17.1
Summary of results of clinical trials investigating sequential, goal-directed therapy
Study | Trial design | Number of patients | Number of weeks | Primary outcome | Subgroup on background therapy outcome |
|---|---|---|---|---|---|
EARLY [7] | DB-RCTa: bosentan vs placebo; 16 % of patients on sildenafil | 185 | 24 | 6MWDb: placebo-adjusted bosentan +19.1 m; P = 0.08 | Subgroup on sildenafil placebo-adjusted 6MWD: −17.3 m (P = 0.85); placebo-adjusted PVR: −22.6 %; P = 0.0478 |
SERAPHIN [8] | DB-RCT: macitentan vs placebo; 62 % on PDE5i, 5 % on inh prostacyclin | 742 | 100 | TTCWc: HR 0.70 for 3 mg macitentan (P = 0.01); HR 0.55 for 10 mg macitentan (P < 0.001) | Subgroup on background therapy: TTCW: HR 0.83 for 3 mg macitentan (P = 0.27); HR 0.62 for 10 mg macitentan (P = 0.009) |
PACES [9] | DB-RCT: sildenafil vs. placebo; all on intravenous epoprostenol | 264 | 16 | 6MWD: Placebo adjusted + 28.8 m (P < 0.001) | All on background therapy by trial design |
Iversen [10] | DB-RCT: all received bosentan; 3 months later 1:1 placebo or sildenafil; 3 months later cross-over to other group | 20 | 12 | 6MWD: bosentan alone + 37 m (P = 0.001); placebo-adjusted addition of sildenafil: +13 m (P = 0.48) | All on background therapy by trial design |
PHIRST [11] | DB-RCT: tadalafil vs placebo; 53 % on background bosentan | 405 | 16 | 6MWD: placebo-adjusted tadalafil 20 mg +23 m (P = 0.09); tadalafil 40 mg +44 m (P < 0.01) | Subgroup on bosentan background therapy: 6MWD placebo-adjusted 40 mg dose +33 m (P = 0.09) |
Imatinib Phase 2 [12] | DB-RCT: imatinib vs placebo; 21 % on mono-background therapy; 65 % on dual-background therapy; 14 % on triple-background therapy | 59 | 24 | (Phase 2) 6MWD: placebo adjusted +23 m (P = 0.21) | All but 1 on background therapy; outcome by number of background medications not reported. |
IMPRES [13] | DB-RCT: imatinib vs placebo; 59 % on dual-background therapy; 41 % on triple background therapy | 202 | 24 | 6MWD: placebo-adjusted +32 m (P = 0.002) | All on background therapy by trial design |
STEP [14] | DB-RCT: iloprost vs. placebo; on background bosentan | 67 | 12 | 6MWD: placebo-adjusted +26 m (P = 0.051) | All on background therapy by trial design |
COMBI [15] | DB-RCT: iloprost vs. placebo; on background bosentan | 40 | 12 | 6MWD: placebo-adjusted -10 m (P = 0.49) | All on background therapy by trial design |
TRIUMPH [16] | DB-RCT: inh treprostinil vs placebo; 70 % on background bosentan and 30 % on sildenafil | 235 | 12 | 6MWD: placebo-adjusted +20 m (P < 0.001) | All on background therapy by trial design |
FREEDOM-C1 [17] | DB-RCT: po treprostinil vs. placebo; 57 % on mono- background therapy; 43 % on dual-background therapy | 354 | 16 | 6MWD: placebo-adjusted +11 m (P = 0.07) | All on background therapy by trial design |
FREEDOM-C2 [18] | DB-RCT: po treprostinil vs. placebo; 59 % on mono- background therapy; 41 % on dual-background therapy | 310 | 16 | 6MWD: placebo-adjusted +10 m (P = 0.089) | All on background therapy by trial design
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|