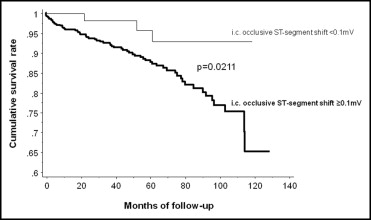
The prognostic relevance of quantitative an intracoronary occlusive electrocardiographic (ECG) ST-segment shift and its determinants have not been investigated in humans. In 765 patients with chronic stable coronary artery disease, the following simultaneous quantitative measurements were obtained during a 1-minute coronary balloon occlusion: intracoronary ECG ST-segment shift (recorded by angioplasty guidewire), mean aortic pressure, mean distal coronary pressure, and mean central venous pressure (CVP). Collateral flow index (CFI) was calculated as follows: (mean distal coronary pressure minus CVP)/(mean aortic pressure minus CVP). During an average follow-up duration of 50 ± 34 months, the cumulative mortality rate from all causes was significantly lower in the group with an ST-segment shift <0.1 mV (n = 89) than in the group with an ST-segment shift ≥0.1 mV (n = 676, p = 0.0211). Factors independently related to intracoronary occlusive ECG ST-segment shift <0.1 mV (r 2 = 0.189, p <0.0001) were high CFI (p <0.0001), intracoronary occlusive RR interval (p = 0.0467), right coronary artery as the ischemic region (p <0.0001), and absence of arterial hypertension (p = 0.0132). “High” CFI according to receiver operating characteristics analysis was ≥0.217 (area under receiver operating characteristics curve 0.647, p <0.0001). In conclusion, absence of ECG ST-segment shift during brief coronary occlusion in patients with chronic coronary artery disease conveys a decreased mortality and is directly influenced by a well-developed collateral supply to the right versus left coronary ischemic region and by the absence of systemic hypertension in a patient’s history.
The goal of this study was to test the hypotheses that a quantitatively obtained occlusive intracoronary electrocardiographic (ECG) ST-segment shift ≥0.1 mV is prognostically hazardous and that the experimentally documented pathophysiologic determinants of the amount of ST-segment shift are corroborated.
Methods
From March 1996 through October 2007, 765 patients (62 ± 10 years old, 580 men) underwent ECG recordings of sufficient quality for quantitative analysis directly before and during coronary balloon occlusion. Coronary angiography was performed for diagnostic purposes. All patients underwent simultaneous recordings of 4-lead surface and unipolar intracoronary electrocardiograms and aortic, distal coronary, and central venous pressures (CVPs) for collateral flow index (CFI) measurement immediately before and during a 1-minute coronary balloon occlusion (first if several occlusions were performed; Figure 1 ). Patients were prospectively selected by the following criteria: (1) no previous Q-wave infarction in myocardial area assessed, (2) no baseline surface ECG ST-segment abnormalities, and (3) absence of acute coronary syndrome. All clinical, ECG, coronary angiographic, and hemodynamic data were prospectively entered in a database, some of which has been described elsewhere.
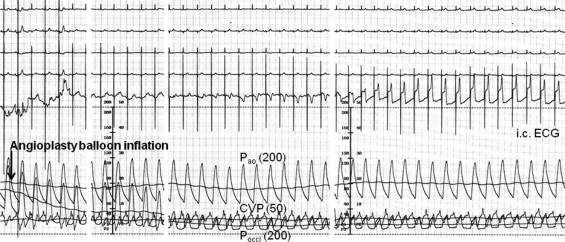
The primary study end point was the quantitatively determined absolute intracoronary ECG ST-segment shift (depression or elevation) at the end of a 1-minute coronary balloon occlusion. Two study groups were formed: patients with occlusive intracoronary ECG ST-segment shift <0.1 mV(n = 89) and those with ≥0.1 mV (n = 676). Secondary end points were the following occlusive intracoronary ECG parameters: ST-segment shift normalized for R-wave amplitude, RR interval, corrected QT interval, T-wave amplitude, and occlusive absolute peripheral ECG (leads II and III) ST-segment shift. Follow-up information on all-cause mortality after study inclusion was obtained by telephone interview of the patients, their relatives, or their family physicians, or by examination of hospital charts.
This investigation was approved by the ethics committee of the Canton of Bern, Switzerland, and patients gave written informed consent to participate in the study.
Quantitatively measured absolute ST-segment shift in intracoronary and surface ECG leads was determined immediately before and at the end of the first 1-minute coronary balloon occlusion ( Figure 1 ). All original thermo-printed ECG curves obtained during cardiac catheterization were electronically scanned and digitized. A customized ECG tracing software package was written in MatLab 7 (MathWorks, Natick, Massachusetts). An interactive interface guided the user through image loading, calibration, and tracing procedures. Fifteen hallmark points were traced on every electrocardiogram including beginning of P wave*, beginning of QRS complex*, peak Q, peak R, peak S, end of QRS complex*, J point, peak T, end of T*, beginning of U, peak U, end of U, beginning of subsequent P*, beginning of subsequent QRS complex*, and subsequent peak R. The points denoted by asterisks were marked and their connection constituted the isoelectric line. If possible, all points on the isoelectric line except end of T were used to fit a straight isoelectric baseline. If some hallmark points were not distinct, definition of the isoelectric line was based on ≥2 hallmark points. Time and voltage calibration lines were drawn along the ECG grid lines on every scan, yielding the basis to convert pixels into seconds and millivolts. Amplification factors of ECG amplitudes were integrated into the calibration process. An angle correction was programmed to compensate for oblique scans and to rectify the voltage/time axes. All traced ECG points were rotated by the amount at which the angle of the time calibration line deviated from horizontal. If the slope of the isoelectric line exceeded 1 or −1, the electrocardiogram was considered excessively undulating and therefore excluded. All amplitudes such as ST-segment shift or R amplitude were calculated as the distance of the corresponding point from the isoelectric line in a perpendicular direction after angle correction. All electrocardiograms were categorized according to intracoronary and surface leads I and II (III, aVR, aVF, aVL, ≥150 missing data points).
Coronary collateral flow relative to normal antegrade flow through the nonoccluded coronary artery (CFI) was determined using coronary pressure measurements ( Figure 1 ). A 0.014-inch pressure monitoring angioplasty guidewire (Pressure Wire, Radi, Uppsala, Sweden) was set at 0, calibrated, advanced through the guiding catheter, and positioned in the distal part of the vessel of interest. CFI was determined by simultaneous measurement of mean aortic pressure (millimeters of mercury), distal coronary artery pressure during balloon occlusion (millimeters of mercury), and CVP (millimeters of mercury). CFI was calculated as (mean distal coronary pressure minus CVP)/(mean aortic pressure minus CVP).
At the start of the invasive procedure, all patients received heparin 5,000 U intravenously. After diagnostic examinations, 2 puffs of oral isosorbide dinitrate were given. The coronary artery judged to be the culprit lesion for a patient’s symptoms was selected for ECG and CFI measurements. This vessel underwent percutaneous coronary intervention (PCI) after ECG and coronary hemodynamic recordings. In the absence of a coronary stenotic lesion, an angiographically and functionally normal coronary artery was selected for occlusive measurement. An adequately sized angioplasty balloon catheter (10 to 20 mm in length, diameter range 2.5 to 4 mm) was positioned in the stenosis to be dilated or at a proximal site in the normal vessel, and the pressure guidewire was positioned distally in the respective vessel. Balloon inflation for ECG and coronary hemodynamic recordings occurred in nonstenotic vessels at a pressure of 1 to 2 atm. Before and during vessel occlusion, an intracoronary electrocardiogram obtained from the guidewire, a 4-lead surface electrocardiogram, mean distal coronary pressure, mean aortic pressure, and CVP were obtained for the calculation of CFI ( Figure 1 ). PCI of the stenotic lesion initially selected was performed immediately after ECG and coronary hemodynamic recordings.
All continuous data are presented as mean ± SD. Patient and invasive characteristics between groups were analyzed by Student’s t tests for continuous data and by chi-square/Fisher’s exact tests for categorical data. Analysis of survival data were performed by Kaplan–Meier analysis. Intraindividual changes of intracoronary ST-segment shift immediately before and during coronary occlusion were analyzed by paired Student’s t test. Linear regression analysis was used for the comparison between occlusive ECG parameters and CFI. Receiver operating characteristics analysis was performed to determine the accuracy and best cutoff of CFI in detecting an occlusive intracoronary ST-segment shift ≥0.1 mV. To assess determinants of occlusive intracoronary ECG ST-segment shift, a forward stepwise multiple regression analysis was performed. Potential determinants of myocardial ischemia during coronary occlusion, i.e., CFI, occlusive intracoronary RR interval, heart rate, previous angina pectoris, coronary vessel undergoing hemodynamic measurements, and cardiovascular risk factors, were entered in the model. Pressure–rate product, a measurement of myocardial oxygen demand, was omitted because of its interdependence with CFI. Instead, heart rate was used. Covariates were centered, and an unweighted effect coding scheme was applied for vessel and risk factor categories.
Results
There were no statistically significant differences between groups for patient age, gender, follow-up duration for survival analysis, and history and classification of angina pectoris ( Table 1 ). There was no difference in frequency of cardiovascular risk factors. Except for use of angiotensin-converting enzyme inhibitors, there was no difference in cardiovascular medication ( Table 1 ).
| Variable | Intracoronary ECG ST-Segment Shift | p Value | |
|---|---|---|---|
| <0.1 mV | ≥0.1 mV | ||
| (n = 89) | (n = 676) | ||
| Age (years) | 61 ± 10 | 63 ± 11 | 0.25 |
| Men | 68 (76%) | 512 (76%) | 0.55 |
| Follow-up duration (months) | 54 ± 36 | 47 ± 34 | 0.07 |
| Angina pectoris | 68 (76%) | 466 (68%) | 0.19 |
| Canadian Cardiac Society class of angina pectoris | 1.7 ± 1.2 | 1.5 ± 1.2 | 0.17 |
| Systemic hypertension | 49 (55%) | 404 (60%) | 0.40 |
| Smoker | 38 (43%) | 236 (35%) | 0.18 |
| Hypercholesterolemia | 59 (66%) | 434 (64%) | 0.83 |
| Obesity (body mass index >30 kg/m 2 ) | 32 (36%) | 275 (41%) | 0.47 |
| Diabetes mellitus | 16 (18%) | 108 (16%) | 0.54 |
| Acetylsalicylic acid | 78 (88%) | 548 (81%) | 0.19 |
| β Blocker | 62 (70%) | 422 (62%) | 0.25 |
| Calcium channel blocker | 19 (21%) | 119 (18%) | 0.40 |
| Nitrate | 27 (30%) | 176 (26%) | 0.51 |
| Statin | 53 (60%) | 361 (53%) | 0.30 |
| Angiotensin-converting enzyme inhibitor | 19 (21%) | 238 (35%) | 0.010 |
| Diuretic | 19 (21%) | 154 (23%) | 0.84 |
Patients with occlusive intracoronary ECG ST-segment shift <0.1 mV showed a lower cumulative rate of all-cause mortality during follow-up than those with ST-segment shift ≥0.1 mV ( Figure 2 ).
Vessels undergoing coronary balloon occlusion for intracoronary ECG and CFI measurements were different between groups: there were more right coronary arteries in the group with ST-segment shift <0.1 mV and more left anterior descending coronary arteries in the group with ST-segment shift ≥0.1 mV ( Table 2 ). Number of vessels with coronary artery disease (CAD) did not differ between groups, nor did mean systemic arterial blood pressure and heart rate before coronary occlusion. Average percent diameter stenosis of the vessel undergoing intracoronary ECG and CFI measurement was more severe in the group with ST-segment shift <0.1 mV. Intraindividual changes of intracoronary ECG ST-segment shift from immediately before to end of coronary balloon occlusion are shown in Figure 3 . The following intracoronary parameters obtained during coronary occlusion were different between study groups ( Table 2 ): ST-segment shift, ST-segment shift normalized for R-wave amplitude, corrected QT interval, and T-wave amplitude; intracoronary RR interval showed a trend to longer duration in the group with ST-segment shift <0.1 mV and this difference was significant when analyzed for the right versus left anterior descending coronary artery (0.934 ± 0.177 vs 0.894 ± 0.177 seconds, respectively, p = 0.0026) and for RR interval difference at the end compared to immediately before occlusion (−0.012 ± 0.098 vs +0.014 ± 0.100 seconds, respectively, p = 0.0002). CFI was higher in the group with occlusive intracoronary ST-segment shift <0.1 mV compared to the group with ≥0.1 mV ( Table 2 ).
| Variable | Intracoronary ECG ST-Segment Shift | p Value | |
|---|---|---|---|
| <0.1 mV | ≥0.1 mV | ||
| (n = 89) | (n = 676) | ||
| Coronary artery undergoing intracoronary electrocardiography for occlusion | <0.0001 | ||
| Left anterior descending | 25 (27%) | 334 (49%) | |
| Left circumflex | 19 (21%) | 201 (30%) | |
| Right | 45 (51%) | 141 (21%) | |
| Number of coronary arteries narrowed | 0.34 | ||
| 0 | 26 (29%) | 232 (34%) | |
| 1 | 29 (33%) | 216 (32%) | |
| 2 | 22 (25%) | 162 (24%) | |
| 3 | 12 (13%) | 69 (10%) | |
| Mean arterial blood pressure (mm Hg) | 94 ± 14 | 93 ± 16 | 0.44 |
| Heart rate (beats/min) | 72 ± 16 | 71 ± 13 | 0.70 |
| Left ventricular ejection fraction (%) | 62 ± 12 | 62 ± 11 | 0.94 |
| Percent diameter stenosis | 55 ± 40 | 47 ± 38 | 0.0469 |
| Intracoronary occlusive RR interval (s) | 0.928 ± 0.191 | 0.897 ± 0.194 | 0.12 |
| Intracoronary occlusive ST-segment shift (mV) | 0.037 ± 0.027 | 0.748 ± 0.552 | <0.0001 |
| Intracoronary occlusive ST-segment shift normalized for R amplitude | 0.026 ± 0.041 | 0.231 ± 0.212 | <0.0001 |
| Intracoronary occlusive corrected QT interval (s) | 0.447 ± 0.006 | 0.459 ± 0.055 | 0.0742 |
| Intracoronary occlusive T amplitude (mV) | 0.099 ± 0.710 | 1.457 ± 1.675 | <0.0001 |
| Collateral flow index (no unit) | 0.249 ± 0.151 | 0.179 ± 0.107 | <0.0001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


