Heart failure (HF) is a common condition in elderly patients. Despite great improvements in medical therapy, HF mortality remains high. Implantable cardioverter defibrillator (ICD) significantly lengthens the survival rate of subjects with severe HF, but little evidence exists on its effect in elderly persons. Aim of this study was to compare the age-related determinants of prognosis in a large population of patients with ICD. We divided all patients who underwent an ICD implantation in 117 Italian centers of the “ClinicalService Project” into 3 age groups (<65, 65 to 74, ≥75 years), and collected clinical and instrumental variables at baseline and during follow-up (median length: 27 months). Between 2004 and 2011, 6,311 patients were enrolled (5,174 men; left ventricular ejection fraction 29% ± 9%); 1,510 subjects were ≥75 years (23.9%; mean age 78 ± 3 years). The prevalence of co-morbidities increased with age. HF was most frequently due to coronary artery disease in the elderly, who also showed the worst New York Heart Association class. At multivariate analysis, older age, coronary artery disease, chronic obstructive pulmonary disease, chronic renal failure, diabetes, complex ventricular arrhythmias, and left ventricular ejection fraction were significant predictors of all-cause mortality. After adjustment, the hazard ratio age group for mortality was 22.6% less than at univariate analysis. When groups were analyzed separately, age alone predicted mortality in the oldest. In conclusion, a large proportion of our population was aged ≥75 years. Mortality was related to age and several co-morbidities, except for the oldest patients in whom age alone resulted predictive.
The prevalence of heart failure (HF) significantly increases with age, affecting over 11% of the subjects aged ≥80 years. Despite advances in medical therapy, mortality remains high in severe forms of disease. Implantable cardioverter defibrillators (ICDs) significantly lengthen the survival rate of subjects with advanced HF. Long-term reduction of mortality is so high that in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) Trial at the 7.6 years follow-up the number needed to treat was 8. The Amiodarone Trialists Meta-analysis showed that the overall proportion of sudden cardiac death (SCD) versus all-cause mortality gradually decreased with age. However, given the large number of elderly patients with HF, the total number of SCDs significantly increased with age. Existing guidelines state that age is not a criterion for withholding ICD implant but advocate caution in the presence of multiple co-morbidities. Elderly patients remain under-represented in controlled clinical trials on the efficacy of ICD. Because of the lack of evidence in this population, clinicians run the risk of underestimating complications and overestimating benefits of this invasive and expensive procedure. The primary end point of this analysis was to identify the determinants of all-cause mortality by age in the Italian ClinicalService Project, a large database of patients who underwent implantation of different types of electrophysiologic devices all over Italy. The secondary end point was to analyze the relation between the MADIT-II score and the prognosis in different age groups.
Methods
As previously reported, since March 2004, 117 Italian cardiology centers have inserted all consecutive patients receiving Medtronic (Minneapolis, Minnesota) implantable cardiac devices in the ClinicalService Project. Data are collected prospectively and may be mined for observational research. An independent physician steering committee identifies key clinical questions on a yearly basis for analysis and publication. The project was approved by each site’s Institutional Review Board or Medical Director and conformed to the principles outlined in the Declaration of Helsinki. Each patient provided written informed consent for data collection and analysis.
We evaluated all patients who received an ICD or a cardiac resynchronization therapy (CRT) device with defibrillator capabilities (CRT-D) between May 2004 and May 2011. Indication for a defibrillator implantation was based on the current guidelines, after optimization of medical therapy, as recommended by the American College of Cardiology/American Heart Association Heart Failure Guidelines. Baseline demographic, clinical, and functional data, a standard electrocardiogram, an echocardiogram, and information on drug therapy were collected for each subject. Follow-up examinations were scheduled according to the clinical practice of each center; therefore, they might have varied in function of the implanted device and clinical conditions. At each follow-up visit, the ICD or CRT-D was interrogated and checked for appropriate functioning, and information on clinical conditions was collected. Mortality data were obtained through telephone interviews to relatives and the treating physician; where possible, the information was validated by consulting medical records.
The primary objective of the study was to evaluate the effect of age on mortality in patients with ICD, and the clinical factors modulating the age-mortality association. Secondarily, we wanted to verify the existence of age-related differences in prognostic predictors.
Patient population was stratified into 3 age groups (<65, 65 to 74, and ≥75 years).
To further analyze the influence of aging on survival rates, we used the MADIT-II score, a tool combining readily available clinical variables indicating the presence of an advanced degree of disease and co-morbidities. The instrument proved effective in identifying ICD recipients at high risk of mortality during follow-up and has already been used for evaluation of a geriatric population. In its original version, one point is assigned for each of the following conditions: the New York Heart Association (NYHA) class >2, age ≥70 years, blood urea nitrogen (BUN) >26 mg/dl, QRS duration >0.12 seconds, and the presence of atrial fibrillation.
Statistical analysis was performed using SAS 9.3 for Windows (SAS Inst. Inc., Cary, North Carolina). Continuous variables are expressed as mean ± SD; twenty-fifth and seventy-fifth percentiles were used to describe the follow-up period. Categorical variables are expressed as raw data and percentages. Different distributions of baseline characteristics were assessed using analysis of variance or chi-square test for continuous or categorical variables, respectively. In univariate analysis, given the large sample size and the existence of the 3 different age groups, statistical significance was attributed for 2-tailed p values <0.017. Survival rates (number of deaths over time) were computed per 100 person-years together with the 95% confidence intervals (95% CIs), and subsequently, compared by means of mixed Poisson models. All-cause mortality was studied using Kaplan-Meier curves and was compared among the groups by means of the log-rank test. Cox regression analysis was performed in univariate models. This allowed reporting the results as hazard ratio (HR) with the related 95% CI. All variables significantly associated with prognosis were introduced into a Cox regression model to identify independent predictors of all-cause mortality. The proportional hazard assumptions were tested by means of Schoenfeld residuals. The presence of a CRT device did not meet the proportional hazard assumption for mortality. Hence, we could not explore the independent weight of the resynchronization therapy on the survival rate in the general model. Consequently, through 2 further Cox regression models, we assessed the effect of age group on mortality in patients with and without CRT. To verify the weight of different clinical predictors of prognosis in subjects aged <65, 65 to 74, and ≥75 years, 3 separate multivariate models, 1 for each age group, were evaluated. In these cases, age was entered as a continuous variable. Finally, on the basis of the demographic composition and the statistical distribution of our population, patients were classified according to the MADIT-II score as at low, intermediate, or high risk for scores ≤1, 2, or >2, respectively. Given that the collection of BUN values was not mandatory, we arbitrarily substituted the variable with the presence of chronic renal failure, condition assigned by each center on the basis of the existing guidelines. For survival rate models and analysis of the MADIT-II score, a 2-tailed p value <0.05 was considered statistically significant.
Results
Of 6,311 patients with ICD, 1,510 (23.9%) were aged ≥75 years. Patients implanted with a CRT-D device were 4,212 (66.7%). Patients’ baseline characteristics are listed in Table 1 . The proportion of men did not vary with age. The prevalence of the most important HF co-morbidities significantly increased in the elderly and in the very elderly, and coronary artery disease (CAD) emerged as the most frequent cause of HF at an advanced age. Left ventricular ejection fraction (LVEF) was similar in all age groups, but very elderly subjects showed lower left ventricular volumes, longer QRS duration, and a more compromised functional profile, as evaluated by the NYHA class. With age, use of β blockers progressively decreased, whereas the use of diuretics and amiodarone increased. In coherence with the age-related correlation between CAD and HF, antiplatelet therapy was more frequent in subjects ≥75 years of age. CRT was more common in elderly and very elderly patients ( Table 1 ).
| Variable | Age Group (yrs) | p Value | ||
|---|---|---|---|---|
| <65 (n = 2,470) | 65–74 (n = 2,331) | ≥75 (n = 1,510) | ||
| Age (yrs) | 55 ± 9 | 70 ± 3 | 78 ± 3 | <0.001 |
| Men | 83.2 | 82.0 | 81.8 | 0.46 |
| AF | 8.1 | 12.6 | 18.3 | <0.001 |
| CAD | 44.3 | 58.2 | 64.7 | <0.001 |
| Chronic obstructive pulmonary disease | 7.6 | 14.1 | 17.0 | <0.001 |
| Diabetes mellitus | 20.6 | 28.3 | 24.8 | <0.001 |
| Hypertension | 45.0 | 59.0 | 60.5 | <0.001 |
| Renal failure | 6.6 | 10.7 | 13.8 | <0.001 |
| Valvular diseases | 16.5 | 19.2 | 16.3 | 0.030 |
| Ventricular arrhythmias | 43.0 | 42.9 | 44.2 | 0.70 |
| Hospital admissions | 49.5 | 50.9 | 49.7 | 0.67 |
| Left ventricular end-diastolic volume (ml) | 218 ± 88 | 202 ± 75 | 183 ± 67 | <0.001 |
| Left ventricular end-systolic volume (ml) | 159 ± 73 | 146 ± 61 | 132 ± 56 | <0.001 |
| LVEF (%) | 29 ± 10 | 29 ± 8 | 29 ± 8 | 0.11 |
| QRS length (ms) | 135 ± 37 | 139 ± 35 | 140 ± 35 | <0.001 |
| NYHA | ||||
| I | 11.0 | 4.2 | 3.8 | |
| II | 43.6 | 40.1 | 36.8 | 0.001 |
| III | 43.2 | 53.2 | 56.8 | |
| IV | 2.3 | 2.5 | 2.7 | |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 81.1 | 80.2 | 77.2 | 0.072 |
| β blocker | 87.8 | 79.8 | 74.0 | <0.001 |
| Diuretic | 94.4 | 96.2 | 96.7 | 0.014 |
| Amiodarone | 43.6 | 49.3 | 49.0 | 0.004 |
| Antiplatelets | 54.6 | 61.4 | 62.6 | <0.001 |
| CRT-D | 62.4 | 70.7 | 67.8 | <0.001 |
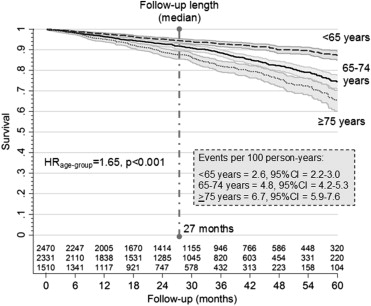
During a median follow-up of 27 months (twenty-fifth to seventy-fifth percentile: 14 to 44 months; on the whole, 16,165 person-years), 685 patients died (4.2 events per 100 person-years, 95% CI 3.9 to 4.6). All-cause mortality was significantly higher in old and very old patients (p <0.001) ( Figure 1 ). Apart from age, univariate analysis showed that male gender, number of hospitalizations, atrial fibrillation, complex sustained ventricular arrhythmias, and co-morbidity were all directly correlated with adverse prognosis. With regard to drug therapy, β blockers appeared to be associated with a protective role on the mortality, whereas diuretics appeared to be associated with an unfavorable outcome ( Table 2 ). Multivariate Cox regression analysis showed that CAD, chronic obstructive pulmonary disease (COPD), diabetes, chronic renal failure, and the presence of ventricular arrhythmias were independent predictors of increased mortality, whereas higher LVEF proved protective ( Table 2 ). After adjustment, the HR value for mortality by age group decreased from 1.65 to 1.58. This result suggests that 22.6% of events in subjects ≥75 years, when compared with those <65 years, could be explained by confounding clinical factors ( Table 2 ). Two Cox multivariate models evidenced that the weight of age group on prognosis was significant both in patients with (HR 1.50, p <0.001) and without (HR 1.86, p <0.001) a CRT device.
| a. Univariate Analysis | HR | 95% CI | p Value |
|---|---|---|---|
| Age group (delta·group) | 1.65 | 1.50–1.81 | <0.001 |
| Gender (men vs women) | 1.42 | 1.13–1.78 | <0.001 |
| AF (yes vs no) | 1.67 | 1.35–2.05 | <0.001 |
| CAD (yes vs no) | 1.68 | 1.43–1.97 | <0.001 |
| Chronic obstructive pulmonary disease (yes vs no) | 1.98 | 1.59–2.47 | <0.001 |
| Diabetes mellitus (yes vs no) | 1.45 | 1.21–1.74 | <0.001 |
| Hypertension (yes vs no) | 1.12 | 0.94–1.33 | 0.21 |
| Renal failure (yes vs no) | 2.27 | 1.79–2.86 | <0.001 |
| Valvular diseases (yes vs no) | 0.98 | 0.78–1.23 | 0.86 |
| Ventricular arrhythmias (yes vs no) | 1.25 | 1.07–1.45 | 0.006 |
| Left ventricular end-diastolic volume (delta·ml) | 1.00 | 1.00–1.00 | 0.26 |
| Left ventricular end-systolic volume (delta·ml) | 1.00 | 1.00–1.00 | 0.14 |
| LVEF (delta·%) | 0.97 | 0.96–0.98 | <0.001 |
| QRS length (delta·ms) | 1.00 | 1.00–1.00 | 0.24 |
| NYHA (III to IV vs I to II) | 1.72 | 1.47–2.02 | <0.001 |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker (yes vs no) | 1.12 | 0.86–1.46 | 0.41 |
| β blocker (yes vs no) | 0.73 | 0.57–0.94 | 0.016 |
| Diuretic (yes vs no) | 3.02 | 1.25–7.30 | 0.014 |
| Amiodarone (yes vs no) | 1.15 | 0.93–1.43 | 0.19 |
| Antiplatelets (yes vs no) | 1.00 | 0.81–1.24 | 1.00 |
| b. Multivariate analysis | HR | 95% CI | p Value |
|---|---|---|---|
| Age group (delta·group) | 1.58 | 1.38–1.80 | <0.001 |
| CAD (yes vs no) | 1.67 | 1.67–1.35 | <0.001 |
| Chronic obstructive pulmonary disease (yes vs no) | 1.55 | 1.18–2.04 | 0.001 |
| Diabetes mellitus (yes vs no) | 1.34 | 1.06–1.68 | 0.013 |
| Renal failure (yes vs no) | 1.63 | 1.23–2.18 | <0.001 |
| Ventricular arrhythmias (yes vs no) | 1.43 | 1.16–1.77 | <0.001 |
| LVEF (delta·%) | 0.97 | 0.96–0.98 | <0.001 |
The 3 different multivariate models analyzing the predictors of mortality for each age group demonstrated that (1) for patients <65 years, only cardiologic variables affected the outcome, (2) for patients aged 65 to 74 years, prognosis was influenced by age, cardiologic variables, and the presence of renal failure, and (3) for patients ≥75 years, age alone exerted a significant influence on the mortality ( Table 3 ).
| a. Univariate Analysis | Age <65 yrs (n = 2,470) | Age 65–74 yrs (n = 2,331) | Age ≥75 yrs (n = 1,510) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age (delta·yrs) | 1.05 | 1.02–1.07 | <0.001 | 1.05 | 1.01–1.10 | 0.016 | 1.10 | 1.05–1.15 | <0.001 |
| Gender (men vs women) | 1.41 | 0.89–2.23 | 0.14 | 1.52 | 1.05–2.21 | 0.028 | 1.36 | 0.94–1.98 | 0.10 |
| AF (yes vs no) | 2.28 | 1.47–3.56 | <0.001 | 1.26 | 0.90–1.76 | 0.17 | 1.33 | 0.95–1.85 | 0.094 |
| CAD (yes vs no) | 1.92 | 1.40–2.62 | <0.001 | 1.53 | 1.19–1.97 | <0.001 | 1.21 | 0.92–1.59 | 0.18 |
| Chronic obstructive pulmonary disease (yes vs no) | 2.18 | 1.31–3.64 | 0.003 | 1.74 | 1.24–2.44 | 0.001 | 1.54 | 1.08–2.19 | 0.016 |
| Diabetes mellitus (yes vs no) | 1.57 | 1.08–2.27 | 0.018 | 1.48 | 1.13–1.9 | 0.004 | 1.15 | 0.83–1.59 | 0.40 |
| Hypertension (yes vs no) | 1.06 | 0.75–1.51 | 0.74 | 1.03 | 0.78–1.35 | 0.85 | 0.95 | 0.71–1.28 | 0.74 |
| Renal failure (yes vs no) | 2.07 | 1.16–3.68 | 0.014 | 2.25 | 1.58–3.20 | <0.001 | 1.71 | 1.18–2.49 | 0.005 |
| Valvular diseases (yes vs no) | 0.93 | 0.58–1.48 | 0.75 | 1.01 | 0.72–1.42 | <0.001 | 1.00 | 0.67–1.49 | 1.00 |
| Ventricular arrhythmias (yes vs no) | 1.12 | 0.82–1.52 | 0.48 | 1.37 | 1.07–1.74 | 0.012 | 1.18 | 0.90–1.54 | 0.24 |
| Left ventricular end-diastolic volume (delta·ml) | 1.00 | 1.00–1.00 | 0.011 | 1.00 | 1.00–1.00 | 0.39 | 1.00 | 1.00–1.00 | 0.51 |
| Left ventricular end-systolic volume (delta·ml) | 1.00 | 1.00–1.00 | 0.24 | 1.00 | 1.00–1.00 | 0.022 | 1.00 | 1.00–1.01 | 0.17 |
| LVEF (delta·%) | 0.97 | 0.95–0.99 | <0.001 | 0.96 | 0.94–0.97 | <0.001 | 0.98 | 0.96–1.00 | 0.015 |
| QRS length (delta·ms) | 1.00 | 1.00–1.00 | 0.096 | 1.00 | 1.00–1.00 | 0.94 | 1.00 | 1.00–1.00 | 0.61 |
| NYHA (III to IV vs I to II) | 1.98 | 1.45–2.71 | <0.001 | 1.71 | 1.32–2.21 | <0.001 | 1.22 | 0.92–1.60 | 0.17 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


