Fig. 10.1
Different delivery routes for cardiac cell delivery
10.2.1 Infusion Delivery
The leading route of delivery in clinical trials (Campbell and Suzuki 2012; Sanganalmath and Bolli 2013) has been through the infusion of a bolus of stem cells into the coronary vasculature with coronary arteries being the main vessels of choice. Systemic intravenous infusion has also been explored.
Intracoronary Infusion
In antegrade methods , the proximal aorta is reached usually by the femoral artery and the target coronary artery is then catheterised. In the clinic, cells are then normally infused under pressure to potentially facilitate ingress into the cardiac tissue. This is achieved by inflating a balloon and infusing the cells distally, thus limiting backflow of cells and also coronary flow forward past the balloon. It is envisaged that this scenario will assist cell delivery by increasing residence time in the infarcted region. Subsequently, several rounds (3–4) of pressurised cellular infusions are then applied. Certainly, this route of delivery is favoured as the access method is one most interventional cardiologists are familiar with, it can be achieved without complex mapping required for catheter based intramyocardial (IM) injections and has the potential to be used in high risk patients due to its minimally invasive nature. Further to this, it may have the potential to aid engraftment, as cells will necessarily tend to reach reasonably perfused cardiac tissue that might be less hostile to cell survival. Of course as a corollary to this, more compromised regions of the infarct may be poorly supplied. Concerns exist regarding the safety of the technique for the delivery of larger stem cells such as mesenchymal stem cells (MSC) wit h reports of pulmonary embolisms resulting from their migration away from the heart (Freyman et al. 2006; Furlani et al. 2009; Vulliet et al. 2004).
Retrograde delivery where access for the percutaneous catheter is gained through the femoral vein via the right atrium, cannulating the coronary sinus and catheterising the target coronary vein has received less attention than the coronary artery route. This method where cells are also infused under pressure avoids issues of occluded arteries precluding access but due the tortuous nature of the venous system is more technically challenging.
Systemic Intravenous Infusion
Intravenous injection has received quite significant interest due to the simple and non-invasive manner through which delivery might be achieved. Successful delivery would be reliant on homing to the cardiac site of injury and this has been demonstrated for cells such as bone marrow mononuclear cells (BMMNC) (Abbott et al. 2004). It is considered to most likely be effective in the context of acute myocardial infarction due to homing signals production being most pronounced at this stage (Frangogiannis 2006). However, it has been calculated based on blood flow rate to the left ventricle that infused cells would have to circulate many times to come into contact with the region of injury allowing time for deposition in other organs (Strauer and Steinhoff 2011).
10.2.2 Intramyocardial Injection
As implied, intramyocardial (IM) injection refers to the most direct way of delivering cells to the injured heart, namely by injection either epicardially, endocardially or through the coronaries. Epicardial delivery is mainly achieved through surgical exposure of the heart and the latter two routes through catheterisation.
Epicardial Delivery
Direct injection into the exposed heart has significant appeal due to the ease with which the injured or scarred region can be visualised and cells precisely delivered to infarcted and/or peri-infarct tissue. Multiple injections with large numbers of cells are also feasible and as with other IM routes, the need for reliance on homing of cells is removed. Due the reduced risk of coronary embolism, larger stem cells such as MSC can be delivered by this route. However, the invasive nature of this approach has generally limited its application clinically to studies where cell delivery is achieved in conjunction with open-heart surgeries such as coronary artery bypass grafting. This unavoidably reduces the number of patients who can be treated in this way. There have been limited reports on the use of minithoracotomies (Klein et al. 2007; Pompilio et al. 2008) to access the heart for direct injection but greater emphasis has been placed on development of catheter based IM injection methods.
Endocardial Delivery
Catheterisation is achieved usua lly through the aortic valve and injections are delivered perpendicularly to the endocardial surface. Placement is typically guided by electromechanical mapping using the NOGA® system (Biosense Webster, Diamond Bar, CA, USA) that allows for visualisation of the infarct and peri-infarct zones (Kharlamov et al. 2013). Thus this approach presents most of the advantages of direct surgical delivery in a minimally invasive manner and has been shown to be feasible and safe (Perin et al. 2003). However, the electromechanical mapping procedure is technically challenging and the equipment expensive.
Trans-coronary Delivery
The coronary sinus is accessed via the venous system with the catheter being passed though the right atrium. Injection is achieved parallel to the direction of the vein and it has been postulated that this may improve engraftment relative to the perpendicular manner in which cells are injected endocardially. The technique has been shown to be feasible and safe with BMMNC in pigs (George et al. 2008) and with skeletal myoblasts in a small clinical trial (Siminiak et al. 2005).
10.3 Engraftment
We have in the main focussed on studies that utilise in vivo imaging techniques to track cellular engraftment within the heart as these approaches allow for longitudinal analysis of cellular fate. They also avoid the potential inaccuracy of histological techniques were only a small fraction of the target area is visualised (Freyman et al. 2006).
The imaging techniques commonly used in cardiac studies (reviewed in (Azene et al. 2014; Chen and Wu 2013; Ransohoff and Wu 2010)) to quantify cellular engraftment exploit either direct physical labelling of the cell population or the introduction of a reporter gene to the cell. Direct labelling has thus far been the method of choice for clinical trials where cellular retention has been analysed, in part because it avoids the alteration of the genetic makeup of the delivered cells with its associated concern for possible mutagenesis. Cells labelled with a suitable contrast agent can then be imaged using the clinically available modalities of positron emission tomography (PET ) and single-photon emission computed tomography (SPECT ) . PET and SPECT have been most widely used, usually with 18F-FDG as a label for the former and 99mTC-HMPAO the latter. PET scanners have a 1–2 order higher sensitivity than SPECT, detecting label at 10−11 to 10−12 mol/L. Cells that have been directly labelled are only really suitable for acute retention studies due to the labels having relatively short half-lives and concerns regarding retention of the label at the site after cell death through for example macrophage engulfment. The use of reporter genes is required for long term tracking as once their stable transfection or transduction into the cell’s genome is achieved, cell signal should be proportional to the number of live cells. The Herpes Simplex Virus type 1 thymidine kinase (HSV1-TK ) and thyroidal sodium iodide symporter (NIS ) are suitable for PET and SPECT respectively when used in conjunction with imaging probes such as [18 F]FEAU (Perin et al. 2011) and 123I (Templin et al. 2012). However, bioluminescence imaging (BLI ) surpasses these techniques in sensitivity by at least 3 orders of magnitude (10−15 mol/L) through the detection of light generated from a reporter gene such as firefly luciferase. This modality at present only finds utility in small animal models due to attenuation of the light as it travels through tissue.
10.3.1 Infusion Studies
There have been a number of clinical studies where acute cellular retention has been assayed for infusion based delivery but not for IM (Table 10.1). The first PET feasibility study was carried out by Hofmann et al. (2005) to assess the retention of autologous BMMNC 75 min after either anterograde intracoronary (IC) or intravenous (IV) delivery to patients who had suffered a myocardial infarction 5–10 days prior. Only 1.3–2.6 % of the 18-F-FDG cells were retained in the heart with IC delivery and none could be detected after IV infusion. The majority of the cells were observed to have accumulated in the liver and spleen. A similar outcome was observed in a slightly larger study of similar design where 17 and 3 patients (3 to >300 days post-myocardial infarction ) were enrolled for IC and IV delivery of peripheral blood stem cells (PBSC), respectively (Kang et al. 2006). Again IV delivery did not result in any detectable levels of cells in the heart and were only found in the lungs 2 h later. 2 % of IC delivered cells remained in the heart with the remainder lodged in the spleen, liver and bone marrow.
Table 10.1
Retention of cell delivery for cardiac repair
Cell type | Model | Route | Duration | Retention | Reference |
|---|---|---|---|---|---|
BMMNC | Human | IC, IV | 75 min | 1.3–2.6 %—IC, no cell detection—IV | Hofmann et al. (2005) |
PBSC | Human | IC, IV | 2 h | 2 %—IC, no cell detection—IV | Kang et al. (2006) |
BMMSC | Canine | IV | 7 days | 0.05 % | Kraitchman et al. (2005) |
BMMNC | Murine | IV | 14 days | 0.1 % | Sheikh et al. (2007) |
BMMNC | Porcine | IC | 1 h | 8.7 %—balloon occlusion, 17.8 %—single-bolus | Doyle et al. (2007) |
BMMNC | Porcine | IC, IM | 24 h | 3.3 %—IC (balloon), 3 %—IC (without), 15 %—IM | Tossios et al. (2008) |
BMMNC | Porcine | IC, IM, RCV | 1 h | 2.6 %—IC, 11 %—IM, 3.2 %—RCV | Hou et al. (2005) |
AdMSC | Porcine | IC, RCV | 1 h and 24 h | 1 h—57.2 %—IC, 17.9 %—RCV | Hong et al. (2014b) |
24 h—22.6 %—IC, 18.7 %—RCV | |||||
BMMNC | Porcine | IC, IM | 1 h | 13,579 cells—IM, 7049 cell—IC | George et al. (2008) |
BMMSC | Rat | IV, IM | 1 week | 15 %—IM, no cell retention—IV | Hale et al. (2008) |
BMMNC | Rat | IV, LV, IM | 24 h | 16 %—IM, <1 %—IV, <1 %—LV | Nakamuta et al. (2009) |
iPSC-CM | Murine | IM | 4 weeks | 8 % | Lepperhof et al. (2014) |
hCPC | Murine | IM | 4 weeks | <5 % | Liu et al. (2012a) |
CSC | Murine | IC | 24 h | 12.7 % | Hong et al. (2014a) |
BMMSC | Porcine | IM | 10 days | 5.8 % | Gyongyosi et al. (2008) |
BMMSC | Porcine | EC | 35 days | 40–50 % | Perin et al. (2011) |
iPSC | Porcine | IM | 15 weeks | ≈2 % | Templin et al. (2012) |
EPC | Canine | EC, IM | 40 min | 57 %—EC, 54 %—IM | Mitchell et al. (2010) |
A variety of animal trials largely substantiate the observations of very poor levels of homing after IV delivery. In a canine study, 111In oxine-labeled bone marrow MSC (BMMSC) were found by SPECT to mainly reach the lungs after 24 h but there was a low level of redistribution to the heart (1.65 % of lung uptake) by 48 h indicating possible homing (Kraitchman et al. 2005). By 7 days as assessed by radioactive counting only 0.05 % of cells infused persisted in the heart. In a mouse BLI study where luciferase expressing BMMNC were injected through the tail vein into either sham operated or ischaemia/reperfusion (I/R) infarcted mice , a significant increase in homing to the infarcted mice’s hearts was observed (Sheikh et al. 2007). However overall levels of engraftment were very low with only 0.1 % of cells residing in cardiac tissue at 14 days post-injection.
The majority of clinical and animal studies examining cell engraftment after IC delivery rendered similar results to the two IC/IV comparative studies described above. Interestingly, in two porcine studies retention of BMMNC was assessed after IC delivery with or without balloon occlusion and found to not significantly differ at either 1 h or 24 h (Doyle et al. 2007; Tossios et al. 2008). This is suggestive that th e homing signals expressed by the injured heart are insufficient to enable cellular egress from the circulatory system in the short period of blocked blood flow before the cells are washed away by the released blood.
Initial comparison of the retrograde and more commonly utilised anterograde IC routes in a porcine model showed no significant difference in cellular engraftment at 1 h with 2.6 % and 3.2 % retention of BMMNC for IC and retrograde coronary venous (RCV) delivery (Hou et al. 2005). Two follow up studies indicate that perhaps IC is slightly more effective than RCV with initially higher levels of adipose derived MSC (AdMSC) engraftment in a porcine model of IC delivery at 24 h though this difference abated at 48 h (Hong et al. 2014b). In a clinical study (Silva et al. 2009), elevated levels of engraftment of 99mTC-BMC were found with IC delivery at 4 and 24 h post administration.
In the majority of the IC, RCV and IV studies, cells were found preferentially located within the pulmonary system. This is of interest as it has been shown that deposition of human MSC into NOD/SCID mice lungs after IV infusion upregulated the anti-inflammatory protein TSG-6 that was then demonstrated to be responsible for a paracrine based reduction in infarct size (Lee et al. 2009).
10.3.2 Intramyocardial Injection
As noted above, thus far no clinical studies have been reported that assay retention and engraftment after intramyocardial (IM) injection. In one of the earlier studies in a porcine model, it was shown that acute retention at 1 h is significantly higher than the infusion methods (IM 11 %, IC 2.6 % and RCV 3.2 %) (Hou et al. 2005). In almost all other direct comparisons between IM and infusion based deliveries, this has been found to be the case (George et al. 2008; Hale et al. 2008; Li et al. 2009, 2011; Nakamuta et al. 2009; Tossios et al. 2008). It is perhaps not entirely surprising as the need for reliance on homing signals to attract cells out of the circulatory system is avoided. However levels achieved though higher than those by infusion are still low and reporter gene studies tend to indicate further rapid loss of cells from the heart. In BLI studies with a range of cell types, 90 % or more of cells that were initially retained are lost in the med ium term (7–28 days post-injection) (Lepperhof et al. 2014; Li et al. 2011; Liu et al. 2012a; Westrich et al. 2010). In addition to these BLI studies, similar decreases in cell numbers in the hear were discerned using a novel real-time PCR analysis of infarcted mouse hearts injected with cardiac derived stem cells (CSC) (Hong et al. 2014a). When autologous porcine BMMSC expressing HSV1-TK and red fluorescent protein (RFP) were injected endocardially via catheterization under NOGA guidance and tracked by PET, signal was detected at 30 h post-injection but the signal was lost at 7 days (Gyongyosi et al. 2008). Though histological analysis for RFP expressing cells indicated around 6 % were still present at 7 days, this is similar to the trends observed in small animals. Very differently in a porcine study utilising HSV1-TK expressing porcine BMMSC where 20-fold greater cell numbers w ere delivered by NOGA guided catheter injection endocardially, an estimated 40–50 % of cells were seen to survive to 35 days in 3 pigs and in one pig to 5 months though with a decreased signal (Chen and Wu 2013; Perin et al. 2011). Interestingly in a porcine study where NIS expressing human iPSC (immunosuppression with cyclosporine A) were injected under NOGA guidance in similarly large numbers either with or without human MSC, only in the pigs that received iPSC injected with MSCs was a signal still detected by SPECT at 15 weeks (Templin et al. 2012). This raises the questions of whether very large numbers of MSC are required to create a more conducive environment for engraftment and whether this is a species-specific effect.
From the above it seems reasonable to assume that endocardial delivery via a catheter would be similar in cellular retention to that achieved with direct epicardial injection. A study in a canine model whereby 111In-tropolone labelled endothelial progenitor cells were either directly injected into the epicardium or endocardial injections were performed using the Stiletto Endo-myocardial Injection System (Boston Scientific) under radio-gr aphic fluoroscopic guidance (Mitchell et al. 2010), equivalent levels of acute retention at 30–40 min post-injection were observed. It would seem that a retention study on IM delivery retention should be performed in humans to ascertain whether the higher, though still underwhelm ing, levels of cells retained with this route in animals are also seen in the clinic.
10.4 Aspects Influencing Cell Retention and Engraftment
Simple mechanical ejection from the delivery site could of course play a role in the immediate loss of cells. Intramyocardial injections of 18-F-FDG labelled CSC into infarcted rat hearts that had either been arrested or had their ventricular rate slowed with adenosine when visualized with PET at 1 h showed substantially improved retention for both conditions (control: 17.8 %; arrested: 75.6 %; adenosine: 35.4 %) (Terrovitis et al. 2009). A similar retention to that achieved with adenosine was seen when the injection hole was simply sealed with fibrin glue. The authors argue that the greater retention seen in the arrested heart may reflect that not only was potential mechanical ejection reduced in this condition but also washout by myocardial perfusion. It might be expected that the mechanical influence on retention would be more pronounced in a heart beating at 300–400 bpm than in the clinic but this has not been empirically determined yet.
An important aspect that might be expected to influence retention and long-term survival would be timing of delivery after infarction. The environment within the infarcted tissue changes dramatically during the remodelling process for many parameters that might influence cells inclusive of inflammation, ischaemia, extracellular matrix structure and biomechanics (Holmes et al. 2005). It is therefore perhaps somewhat surprising that in the relatively limited number of studies directly examining the consequence of timing, no marked effect was observed. In a human study where retention of 111IN-oxine labelled PBSC was assessed by SPECT, 6.3 % acute retention was observed in infarcts less than 14 days old relative to the 4.5 % in older infarcts (Schachinger et al. 2008). Small animal studies have seen similar results with little or no difference in retention observed whether cells are injected acutely or at later time points (Bonios et al. 2011; Nakamuta et al. 2009; Swijnenburg et al. 2010).
In a recent meta-analysis of stem cell therapy outcome in the clinic, a positive correlation between mononuclear cell dose infused and increase in ejection fraction was detected (Clifford et al. 2012). The limited studies that have assessed the impact of dosage on retention and survival have also found a similarly positive relationship. 1–2 % of either 105 or 106 BMMSC or BMMNC were found to survive at 6 weeks after injection into rat hearts (Muller-Ehmsen et al. 2006) and acute retention (Shen et al. 2012) was found to be around 10 % for all dosages (104 to 5 × 105) of cardiospheres (a natural mixture of resident cardiac stem cells and supporting cell types (Smith et al. 2007)) with a positive correlation between dosage and functional recovery. An elegant study by Liu et al (Liu et al. 2012a) took advantage of the apparent variability present in epicardial intramyocardial delivery (Hou et al. 2005) to stratify their treatment cohort (HSV1-TK expressing human cardiac progenitor cell (hCPC) delivered into infarcted SCID mice hearts) into high and low early engraftment groups. A clear and significant improvement in various left ventricular (LV) functional parameters was observed for the high engraftment group, which was ascribed to the paracrine effect.
These latter two studies emphasize that improving retention and survival of stem cells delivered will correspond to improved outcome. We shall devote the remainder of this chapter to exploring the utilization of injectable biomaterial scaffolds to achieve this desired improvement. Biomaterials are attractive as they in their various guises afford the possibility of tackling multiple factors that might influence engraftment such as mechanical entrapment and reduction of anoikis, inflammation and ischaemia.
10.5 Injectable Biomaterial Cellular Vehicles
10.5.1 Biomaterial Injection
Clearly, to act as cellular delivery vehicles injectable biomaterial scaffolds should gel sufficiently quickly after injection to effectively entrap cells, they must be biocompatible and allow for cellular adhesion to reduce anoikis. The ability to stimulate potentially cellular protective mechanisms such as angiogenesis would of course be desirable. These and other related aspects are the subjects of intensive recent research as will be seen below. However, injectable materials can be intrinsically cardioprotective. The cyclical process of pathological left ventricular dilation that ensues after a myocardial infarction is driven by increased stress in the wall (Opie et al. 2006; Sutton and Sharpe 2000). This increased stress can be considered to derive from the interaction between the increasing ventricular volume and the thinning of the infarcted wall as described by the Law of Laplace 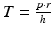 , where T is the tension in the cardiac wall, p is the blood pressure in the ventricular cavity, r is the radius of the ventricular cavity and h is the thickness of the cardiac wall. As can be seen from this, an increase in wall thickness will reduce the stress experienced by the cellular components of the ventricular wall and thus potentially inhibit the progression towards heart failure. Injection of a material within the wall can achieve such thickening and finite elem ent models (Wall et al. 2006; Wenk et al. 2009) have shown that this resultant bulking can reduce stress by up to 20 % in the critical border zone region and slightly increase the ejection fraction (EF) .
, where T is the tension in the cardiac wall, p is the blood pressure in the ventricular cavity, r is the radius of the ventricular cavity and h is the thickness of the cardiac wall. As can be seen from this, an increase in wall thickness will reduce the stress experienced by the cellular components of the ventricular wall and thus potentially inhibit the progression towards heart failure. Injection of a material within the wall can achieve such thickening and finite elem ent models (Wall et al. 2006; Wenk et al. 2009) have shown that this resultant bulking can reduce stress by up to 20 % in the critical border zone region and slightly increase the ejection fraction (EF) .
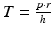 , where T is the tension in the cardiac wall, p is the blood pressure in the ventricular cavity, r is the radius of the ventricular cavity and h is the thickness of the cardiac wall. As can be seen from this, an increase in wall thickness will reduce the stress experienced by the cellular components of the ventricular wall and thus potentially inhibit the progression towards heart failure. Injection of a material within the wall can achieve such thickening and finite elem ent models (Wall et al. 2006; Wenk et al. 2009) have shown that this resultant bulking can reduce stress by up to 20 % in the critical border zone region and slightly increase the ejection fraction (EF) .
, where T is the tension in the cardiac wall, p is the blood pressure in the ventricular cavity, r is the radius of the ventricular cavity and h is the thickness of the cardiac wall. As can be seen from this, an increase in wall thickness will reduce the stress experienced by the cellular components of the ventricular wall and thus potentially inhibit the progression towards heart failure. Injection of a material within the wall can achieve such thickening and finite elem ent models (Wall et al. 2006; Wenk et al. 2009) have shown that this resultant bulking can reduce stress by up to 20 % in the critical border zone region and slightly increase the ejection fraction (EF) .The above finite element modelling findings have been widely supported by studies that have looked at injection of a broad range of biomaterials into infarcted hearts, inclusive of both natural materials (e.g fibrin; collagen; matrigel; extracellular matrix derivatives; alginate) and synthetic (e.g. self-assembling peptides; polyethylene glycol; poly(N-isopropylacrlamide) polymers) (reviewed in Nelson et al. 2011, Radisic and Christman 2013). These studies have on the whole observed wall thickening and varying degrees of functional preservation. There is a paucity of studies that have empirically in vestigated the mechanism through which these above results were obtained. However, a recent study (Ifkovits et al. 2010) demonstrated as predicted by the mathematical models, a stiffer methacrylated hyaluronic acid hydrogel resulted in less infarct expansion and left ventricular dilation in a porcine infarction model. This type of research is urgently needed to guide the design and optimisation of injectable hydrogels for cardiac therapy. It should be noted that the above finding also raises potential further complexity in development of these hydrogels as cellular vehicles because the stiffness of hydrogels can significantly influence the behaviour of stem cells entrapped within them—both with respect to their ability to migrate within the hydrogel (Ehrbar et al. 2011) and their potential direction of differentiation (Pek et al. 2010).
As a stand-alone cardiac therapy, alginate has been the most intensively investigated. Alginate, an anionic polysaccharide derived from br own seaweed is biocompatible and widely used in the pharmaceutical and medical device industries. A low viscosity version of alginate has been developed that is injectable and polymerises spontaneously within the heart due to increasing calcium ion concentration in the infarct. Landa et al. (2008) investigated the effects of alginate delivery via epicardial injection into rat hearts 7 and 60 days after infarct induction. The alginate was gradually replaced by connective tissue over 6 weeks demonstrating biodegradability. It should be noted that though a non-degradable implant mi ght be considered desirable as stress reduction might also be maintained, we and others have shown this to not be the case even when using very biocompatible polyethylene glycol (PEG) hydrogels (Dobner et al. 2009; Rane et al. 2011). Eight weeks after injection of alginate, infarct scar thickness was increased and left ventricle dilation reduced for injections at both time points though these improvements were diminished in the group receiving injections into the chronic infarct. The latter result emphasising the greater difficulty treating infarcted hearts at that late stage. Of interest, the positive outcomes in the 7 day injection group were at least comparable to a group that received 1 × 106 neonatal cardiomyocytes in saline. In a follow up large animal study, the alginate solution was delivered to infarcted porcine hearts at 4 days post-infarction and again reductions in ventricular dilation and increased scar thickness were observe d (Leor et al. 2009). Interestingly, intracoronary delivery was realized through catheter injection within the left anterior descending artery and ingress into the infarcted tissue was achieved through the leaky vessels present in the infarct. Occlusion of the coronary was avoided, as calcium levels were only high enough within the tissue to achieve phase inversion to a hydrogel. This relatively simple minimally invasive approach is certainly appealing for clinical application of alginate as a stand-alone therapy but with respect to use as a route for enhancing cellular engraftment it may be less effective. The probable inefficient migration of cells from the vasculature into the myocar dium that blights intracoronary delivery is likely not to be improved by delivery with a hydrogel.
These promising pre-clinical results with alginate resulted in an initial feasibility and safety trial in humans (ClinicalTrials.gov Identifier NCT00557531) whereby 2 mL of the alginate solution (termed IK-5001) was delivered via the infarcted coro nary to 27 patients that had undergone a moderate to large myocardial infarction 7 days prior. The patients had been successfully revascularised. The treatment was well tolerated and preservation of LV indices was observed (Frey et al. 2014). This positive outcome has resulted in the enrolment of 300 patients with acute myocardial infarction into an ongoing multicentre, randomized and double-blind phase 2 clinical trial (ClinicalTrials.gov Identifier NCT01226563). In a related human study, an alternative form of alginate was delivered by epicardial intramyocardial injection in 10–15 sites in 11 dilated cardiomyopathy patients that were undergoing coronary artery bypass grafting (CABG) (ClinicalTrials.gov Identifier NCT00847964). In a small subset that could undergo MRI, it was determined by mathematical modelling that myofibre stress was reduced by 35 % (Lee et al. 2013). So though this outcome derives from only three patients and is complicated by the simultaneous CABG procedure, it is the first indication that hydrogel based stress reduction is achievable in humans.
The progression of these types of therapies to the clinic will be facilitated by their ability to be delivered by catheterization. This is a demanding goal as the polymer solutions need to have low enough viscosity to flow through the catheter and remain ungelled till reaching their target site upon which gelation must occur as rapidly as possible. Apart from alginate (see above), there are very few reports describing catheter delivery of a hydrogel to the heart. Recently though a hydrogel solution derived from ventricular extracellular matrix was successful injected endocardially into porcine hearts (Singelyn et al. 2012) using a NOGA® guided Myostar® catheter. In follow up study, the catheter delivered extracellular matrix hyd rogel was shown to improve cardiac function in a porcine myocardial infarct model (Seif-Naraghi et al. 2013). More recently a pH-switchable hydrogel (ureido-pyrimidinone-modified PEG hydrogel) was used to deliver growth factors to infarcted porcine hearts (Bastings et al. 2014). Th ough probably not useful for cellular delivery due to the switch occurring as the solution at pH 8.5 transits to neutral in the heart, the type of rapid and controllable gelling achieved will be d esirable for cellular vehicles.
These types of outcomes with biomaterial delivery alone have resulted in significant recent interest in determining the influence of co-d eliver y of injectable hydrogels with stem cells on both stem cell retention and efficacy .
10.5.2 Biomaterial-Cell Delivery
Biological Materials
Biological materials are inherently attractive as cellular vehicles as they usually have a good level of biocompatibility. They are also readily available but have batch-to-batch variability that can be eliminated in synthetic hydrogels.
Fibrin, a well characterised hydrogel that is derived from the mixing of a solution of fibrinogen with one containing thrombin, factor XIIIa and calcium, was one of the earliest materials used to deliver cells to the heart (Table 10.2). In the initial studies, skeletal myoblasts were delivered with fibrin to infarcted rat hearts. The combination and controls were injected 1 week post-infarction and hearts were assessed by echocardiography and histology 4 weeks later (Christman et al. 2004a, b). As assessed by skeletal myosin immunohistochemistry, engraftment was similar for the cell and cell plus fibrin groups at 24 h but by 4 weeks post-injection a twofold increased engraftment of skeletal myoblasts was seen in cells plus fibrin. Similar functional improvements were seen for fibrin, cells and cells plus fibrin and all 3 groups were seen to elicit an angiogenic response. Thus in this instance the simpler route of only delivering the material without the skeletal myoblasts would appear optimal. In another fibrin based study, 99mTc-labelled BMDC were quantified 24 h after injection into infarcted rat hearts through counting the radioactivity in the excised organ in a gamma counter (Nakamuta et al. 2009). In this study, using a more global means of engraftment assessment, a 2.5-fold increase in cell retention was seen for the fibrin plus cells group. Again at 4 weeks post-infarction for both the cell alone and cell plus fibrin groups similar functional improvements were observed though with a trend towards greater scar thickness in the latter. In a BLI based analysis of retention of luciferase expressing AdMSC with or without fibrin injected into infarcted rat hearts, an increasing divergence between cells alone and cells with fibrin was quantified (1 day: 1.3 ×; 7 days: 3 ×, 14 days: 5 ×) till finally at day 28, a signal could readily be detected for fibrin plus cells but none for cells alone (Yang et al. 2013). It should be noted that though there was greater engraftment for fibrin plus cells, there was still a rapid decrease in signal with only about 2 % of the original 5 million AdMSC present in the heart. Finally with respect to fibrin, in a follow up to the study of Nakamuta et al. (2009), the functional recovery after AdMSC delivery to infarcted hearts was assessed in much greater detail (Danoviz et al. 2010). Stroke work measured in the presence of phenylephrine, a global index of cardiac function that depends on both pressure generation and ejection capability was seen to be improved by all cell containing groups but only returned to normality when hearts were injected with a biomaterial plus cells. There was a positive correlation between greater cellular engraftment ((AdMSC/media: 4.8 %; AdMSC/fibrin: 13.8 %; AdMSC/collagen 26.8 %) measured by 99 m-Tc labeling) and stroke work improvement.
Table 10.2
Cell delivery within biomaterials for cardiac repair
BMMSC < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|