Delayed Enhancement in Nonischemic Myocardial Disease
Charles B. Higgins
Karen G. Ordovas
GOALS AND INDICATIONS
The major goals and indications for late gadolinium enhancement (LGE) magnetic resonance (MR) in nonischemic heart disease are:
Diagnosis of acute and chronic myocarditis
Recognition of infiltration and bulk fibrosis in cardiomyopathies
Risk assessment and prognosis in dilated and hypertrophic cardiomyopathies
Diagnosis and risk assessment in cardiac sarcoid
Diagnosis of arrhythmogenic right ventricular dysplasia (cardiomyopathy)
Recognition of myocardial involvement in systemic vasculitides
Recognition of myocardial involvement in chronic infections
Recognition of myocardial fibrosis after repair of Tetralogy of Fallot
LGE occurs in many myocardial diseases other than ischemic heart disease (1,2). While the distribution of LGE is invariably subendocardial for acute and chronic myocardial infarction, the pattern of enhancement has a variable transmural distribution in nonischemic myocardial disease. LGE conforms to the distribution of one or more coronary arteries in ischemic heart disease while in nonischemic myocardial disease, it does not. With nonischemic myocardial disease the mural pattern is not uncommonly midwall or subepicardial and may be spotty in multiple regions of the ventricle. The pattern of distribution in some may be almost diagnostic of a specific myocardial disease. In some nonischemic myocardial disease the mere presence as well as the overall extent of LGE carries predictive implications for morbidity and mortality.
MECHANISM OF LGE IN NONISCHEMIC MYOCARDIAL DISEASE
The mechanism of LGE in acute and chronic infarction is the increase in the extracellular space caused by necrosis (loss of cell membrane integrity) with the former and the larger extracellular space of scar compared to myocardium with the latter (1). The mechanism in nonischemic myocardial disease is also predominantly, if not exclusively, due to increase in the distribution volume of gadolinium chelates
due to an increase in the extracellular space of the myocardium (1). The increase in the extracellular space may be due to necrosis of myocardial cells (myocarditis), replacement of myocardial cells by bulk fibrosis (hypertrophic cardiomyopathy [HCM]), or replacement of myocardium by various infiltrates (amyloidosis, Anderson-Fabry disease, sarcoidosis), or a combination of two or three of the above (1).
due to an increase in the extracellular space of the myocardium (1). The increase in the extracellular space may be due to necrosis of myocardial cells (myocarditis), replacement of myocardial cells by bulk fibrosis (hypertrophic cardiomyopathy [HCM]), or replacement of myocardium by various infiltrates (amyloidosis, Anderson-Fabry disease, sarcoidosis), or a combination of two or three of the above (1).
VIRAL MYOCARDITIS
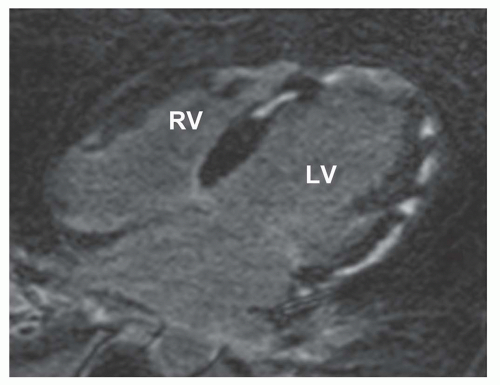
Some but not all patients with acute or subacute myocarditis show LGE (3,4,5,6,7 and 8) (Fig. 18.1). In acute myocarditis there is invariably myocardial hyperenhancement on T2-weighted imaging even when no LGE is evident (3). Moreover, LGE may ensue later than T2 hyperintensity and persist after resolution of T2 hyperenhancement. T2 hyperenhancement is attributed to myocardial edema while LGE indicates myocardial necrosis and/or fibrosis. LGE is detected in about 70% of patients with chronic myocarditis (7,8).
The location of LGE in myocarditis is subepicardial (38%) or midwall (68%) in distribution in either ventricle or both (3,4,5 and 6). Some etiologic agents of viral myocarditis may favor a specific location in the ventricle(s) such as the posterolateral wall of the left ventricle with parvovirus (6) (Fig. 18.2).
DILATED (CONGESTIVE) CARDIOMYOPATHY
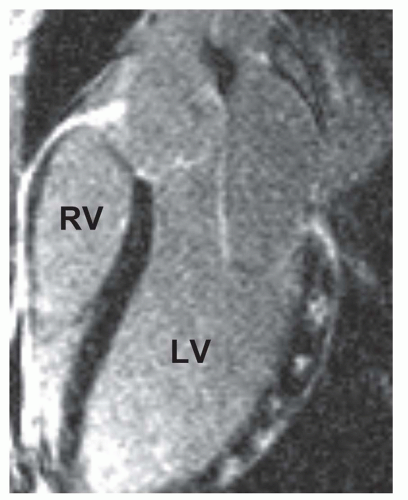
LGE is used to distinguish between ischemic-dilated and nonischemic-dilated cardiomyopathy. Subendocardial or transmural LGE is indicative of ischemic etiology of a dilated cardiomyopathy. Idiopathic-dilated cardiomyopathy usually has no LGE (Fig. 18.3). On the other hand, LGE is observed in about 30% of patients with alleged nonischemic-dilated cardiomyopathy (9). The distribution is usually midwall rather than subendocardial (9).
The extent of fibrosis depicted on LGE images has been associated with increased intraventricular dyssynchrony in dilated cardiomyopathy (10). Patients with LGE have been shown to have more future adverse cardiac events (11). The presence and extent of LGE was more predictive of adverse cardiac events than left ventricular ejection fraction (11,12).
LEFT VENTRICULAR PARTIAL NONCOMPACTION
Partial noncompaction is now recognized as the cause of dilated cardiomyopathy especially in older children and young adults. It is classified as a primary genetic-dilated cardiomyopathy. The left ventricular volume is increased and ejection fraction reduced. The diagnostic criterion on MR is established on the cine MR image at end diastole in the short-axis phase. A ratio of noncompacted to compacted wall thickness greater than 2.3 establishes the diagnosis. It is not yet clear if lesser ratios identify milder forms. LGE of the trabecular portion has been reported for more severe or late form of the disease (13). Because of stasis of gadolinium-enhanced blood pool within the trabecula, interpretation of this finding may be equivocal.
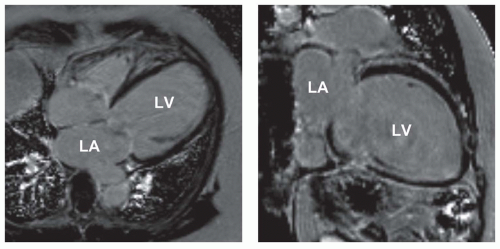 Figure 18.3. Idiopathic-dilated cardiomyopathy. LGE image in HLA (left) and VLA (right) planes shows severe LV enlargement and no delayed hyperenhancement at 15 minutes after injection of gadolinium chelate. LA, left atrium; LV, left ventricle. (From Kim RJ, Shah DJ, Mudd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson 2003;5(3):505-514, with permission.) |
PERIPARTUM-DILATED CARDIOMYOPATHY
Peripartum-dilated cardiomyopathy is diagnosed when heart failure and dilated left ventricle with reduced ejection fraction ensues in the last month of or first 5 months after delivery in a previously healthy woman in the absence of an identifiable etiology. Cine MR shows increases in LV enddiastolic and end-systolic volumes and decreased ejection fraction. In a minority of patients, LGEs have been identified in a midwall distribution (14).
HYPERTROPHIC CARDIOMYOPATHY
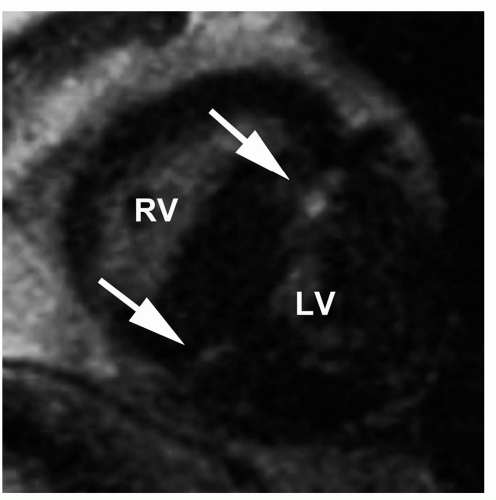
Cardiac MR imaging is important in assessing the severity and distribution of hypertrophy in HCM. It is the most effective modality in the latter, especially in disclosing unusual hypertrophy patterns such as midventricular and apical forms of HCM. Moreover, the quantification of LV mass and identification of LGE by CVMR constitute two of the most important prognostic indicators in this disease (15). LGE usually corresponds to areas of most severe hypertrophy with focal necrosis and/or fibrosis (16).
LGE has been repeated in 81% of patients with HCM (17). The distribution is focal in the area of most severe hypertrophy, usually the ventricular septum (Fig. 18.4), or at the junction point between the septum and the anterior and posterior walls of the ventricle (17) (Fig. 18.5). It is usually midwall in distribution and never subendocardial.
LGE is important in risk assessment in patients with HCM. Patients with HCM and any degree of LGE have a sevenfold higher risk of nonsustained ventricular tachycardia (18). In addition to an increased incidence of arrhythmias, LGE is also associated with poorer functional class, decreased left ventricular function, conduction disturbances, abnormal Q-waves, and giant T-waves (19). Patients with more rapid progression of disease as reflected in left ventricular dilatation have more extensive LGE (15). The same study showed greater extent of LGE in patients with two or more risk factors for sudden death. Thus, CVMR with LGE imaging is probably indicated in all patients with HCM not necessarily for diagnosis but for risk assessment and prognosis.
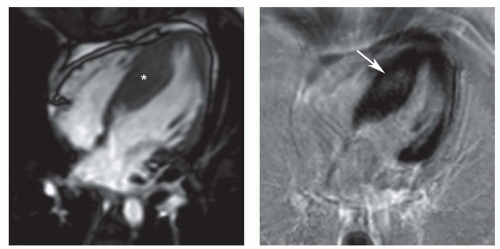 Figure 18.4. Hypertrophic cardiomyopathy. Cine MR (left) and LGE (right) images show asymmetric septal hypertrophy (asterisk). The hyperenhancement (arrow) is at the site of maximum septal hypertrophy. (From Kim RJ, Shah DJ, Mudd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson 2003;5(3):505-514, with permission.) |
RESTRICTIVE CARDIOMYOPATHY
Restrictive cardiomyopathy (RCM) is characterized typically on CVMR by dilated atria in the presence of normalor small-sized ventricles. Functionally, there is diastolic dysfunction with normal or only mildly reduced left ventricular systolic function. Some cases of RCM are due to myocardial infiltrative diseases or storage diseases. The infiltrative types sometimes can be recognized by the presence of even a pathognomonic pattern of LGE such as that occurs in cardiac amyloidosis. The LGE in these entities is described below under the several specific entities. Other causes of RCM involve fibrosis of the myocardium. These entities may or may not demonstrate LGE.
SPECIFIC INFILTRATIVE CARDIOMYOPATHIES
CARDIAC AMYLOIDOSIS
Cardiac amyloidosis is characterized on echocardiography and cine MR by thickened myocardial walls (usually >15 mm) and reduced left ventricular ejection fraction. The differential diagnoses are usually compensatory left ventricular hypertrophy and HCM. Both of the latter have high normal or supranormal left ventricular ejection fraction. The diagnosis of cardiac amyloidosis can be made with a sensitivity of 80% and specificity of 94% with DCE-MR imaging using myocardial biopsy as the gold standard (20). Global subendocardial pattern of DCE is nearly pathognomonic for amyloidosis (20




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree