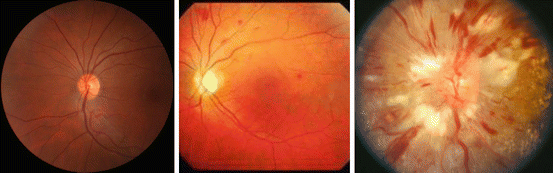
Grade
Features
1
Mild generalized retinal arteriolar narrowing
2
Definite focal narrowing and arteriovenous nipping
3
Signs of grade 2 retinopathy plus retinal hemorrhages, exudates, and cotton wool spots
4
Severe grade 3 retinopathy plus papilledema
11.1.1 Funduscopy
In the last decade several approaches have been developed, assessing more sensitive and quantitative alterations of retinal microvascular changes. Although protocols may differ in some minor points, the principles are similar. According to standardized protocols, one (e.g., 45°) nonstereoscopic color retinal photograph centered between the optic disk and the macula and approximately two disk diameters nasal to the optic disk has to be done in a darkened room. Hence, due to dark adaption, mydriatic agents are no longer necessary. However, in some studies (e.g., Rotterdam Study), pharmacological mydriasis was routinely done. For quantitative assessment of retinal vessels, the photographs have to be converted to digital pictures and analyzed by specific imaging software, e.g., the “Interactive Vessels Analysis” (IVAN) (University of Wisconsin, Madison, WI, USA). This software analysis provides semiautomated measurement of retinal arterioles and venules. Using formulas (e.g., Parr and Spears [8] or Knudtson et al. [9]), a single “central retinal artery equivalent (CRAE)” and a “central retinal vein equivalent (CRVE)” are calculated. Subsequently, arteriole-to-venule ratio (AVR) can be computed; for details see Hubbard et al. [10]. However, by this method, it is not possible to evaluate the retinal vascular wall thickness or vessel diameter directly.
11.1.2 Scanning Laser Doppler Flowmetry
SLDF, introduced by our study group about 10 years ago, allows the dynamic assessment of both functional (i.e., vascular tone) and structural parameters (i.e., wall and lumen diameter). In brief, SLDF is performed in the juxtapapillary area of the right eye, 2–3 mm temporal superior of the optic nerve at 670 nm (Heidelberg Retina Flowmeter, Heidelberg Engineering, Germany). A retinal sample of 2.56 × 0.64 × 0.30 nm is scanned within 2 s (at least one full systolic and one diastolic phase) and measured every 10 μm of this specific length of the retinal arteriole (80–140 μm). The confocal technique of the device ensures that only capillary flow of the superficial layer of 300 μm is measured. No pupil dilation is necessary (i.e., no constriction of patient daily routine) [11].
For assessment of functional parameters, mean retinal capillary flow (RCF) is assessed in the area of interest, and for further dynamic analysis, non pharmacological and pharmacological tools can be applied. Flicker light increases RCF at least in part via a nitric oxide (NO)-dependent mechanism and represents a non pharmacological tool to investigate vasodilatory capacity of retinal arterioles. It is noteworthy to mention that flicker light exposure has no effects on systemic blood pressure (BP), thereby minimizing potential systemic hemodynamic influences on RCF. Moreover, basal NO activity can be assessed by administration of the NO synthase inhibitor NG-monomethyl-l-arginine (l-NMMA).
For assessment of structural parameters, the outer arteriole diameter (AD) is measured by reflection images, and the lumen diameter (LD) is measured by perfusion images. From the raw parameters, wall thickness (WT, [AD−LD]/2), WLR ([AD−LD]/LD) (Fig. 11.2), and wall cross-sectional area (WCSA, п/4 × [AD2–LD2]) can be calculated.
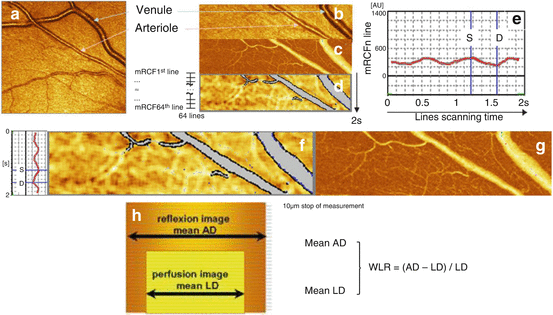

Fig. 11.2
Scanning laser Doppler flowmetry (SLDF) (Republished from Ott and Schmieder [2]). (a) Differentiation between retinal arteriole and venule (SLDF live image before measurement). (b) Scanned area – reflection image. (c) Scanned area – perfusion image. (d) Scanned area – corrected and analyzed flow image. (e) Pulse curve run as mean retinal capillary flow (RCF) and time plot. (f) Localization of systolic and diastolic RCF on the image (d). (g) Localization of systolic and diastolic RCF on the image (c). (h) Calculation of wall-to-lumen ratio
Importantly, also individual pulsatile pattern of functional (RCF) and structural (e.g., WT) parameters of retinal arterioles in systole and diastole can be reliably assessed (Fig. 11.2).
All analyses are performed offline with automatic full-field perfusion imaging analysis (AFFPIA) (SLDF Version 4.0 by Welzenbach with improved resolution) [11].
11.2 Prevalence and Incidence (General Population, Hypertension)
11.2.1 Funduscopy
Several population-based studies have provided data on prevalence of retinal signs using standardized funduscopic photographs in the general population, partly with subsequent categorization according to (among others) hypertension status. In general, retinal signs are common in people aged 40 years or older, even in those without arterial hypertension. However, these findings can only be respected with caution, since different definitions of arterial hypertension have been used. Moreover, the reported prevalence of retinal signs depends also largely on the assessed parameter, e.g., retinopathy per se, AV nicking, or focal/generalized arteriolar narrowing (for details see Table 11.2).
Table 11.2
Prevalence/incidence of retinal signs in normotensive and hypertensive populations using funduscopy
Study | Ref. | Ethnicity | Year | Sample | Age | FU | BP definition | Retinal signs | Prevalence | Incidence normo-/hypertensive people |
|---|---|---|---|---|---|---|---|---|---|---|
London, England | [12] | White (Afro-Caribbean) | 1995 | 1,164 | 40–64 | – | ≥160/95 mmHg or use of drugs | Keith classification | 8 (13)/32 (31) (men) | |
8 (20)/18 (28) (women) | ||||||||||
Beaver Dam Eye Study (BDES) | [13] | White | 1997 | 3,115 | 43–86 | 5 | ≥160/95 mmHg or use of drugs | Focal art. narrowing | 7.7/15.2 | |
Retinopathya | 4.6/9.2 | |||||||||
Blue Mountains Eye Study (BMES) | [14] | White | 1998 | 3,275 | ≥49 | – | ≥160/95 mmHg or use of drugs | Retinopathya | 8.6/12.5 (men) | |
7.4/12.6 (women) | ||||||||||
[15] | 2006 | 1,725 | 5 | ≥140/90 mmHg or use of drugs | Retinopathya | 8.2/10.4 | ||||
Cardiovascular Health Study (CHS) | [16] | White, black | 2003 | 2,050 | ≥65 | – | ≥140/90 mmHg or use of drugs | Retinopathya | 4.7/7.5 (men) | |
6.3/11.9 (women) | ||||||||||
Focal art. narrowing | 5.3/7.2 (men) | |||||||||
6.7/15.0 (women) | ||||||||||
AV nicking | 5.8/8.0 (men) | |||||||||
6.0/9.6 (women) | ||||||||||
General. art. narrowing | 19.7/30.0 (men) | |||||||||
(Lowest 20 % of AVR) | 14.3 /23.0 (women) | |||||||||
Atherosclerosis Risk in Communities (ARIC) Study | [17] | White, black | 2003 | 9,734 | 51–72 | – | ≥140/90 mmHg or use of drugs | Retinopathya | 3.6/5.3 (whites) | |
5.9/9.1 (blacks) | ||||||||||
Hoorn Study | [18] | White | 2003 | 176 | 50–74 | 9.4 | ≥160/95 mmHg or use of drugs | Retinopathya | 6.1/20.0 | |
Beijing Eye Study | [19] | Chinese | 2009 | 3,322 | ≥40 | – | ≥140/90 mmHg or use of drugs | Focal art. narrowing | 6.2/12.1 | |
AV nicking | 6.1/12.3 | |||||||||
General. art. narrowing (lowest 25 % of AVR) | 14.6/25.4 |
In the Cardiovascular Health Study (CHS) (aged ≥65 years), 16.6 % (men 19.7 %; women 14.3 %) of normotensive participants and 25.4 % (men 30.0 %; women 23.0 %) of hypertensive patients (defined as BP ≥140/90 mmHg or history of hypertension with use of antihypertensive drugs) were reported to have generalized arteriolar narrowing (defined as the lowest twentieth percentile of AVR). In contrast, retinopathy was by far less frequently documented in this study, i.e., in 5.6 % (men 4.7 %; women 6.3 %) of normotensive participants and in 10.4 % (men 7.5 %; women 11.9 %) of hypertensive patients [16]. Confirmatory results were found in the Beijing study (aged ≥ 40 years), which also used the accepted criteria of hypertension (BP ≥140/90 mmHg or history of hypertension with use of antihypertensive drugs) [19]. Moreover, in the former study another important point was found, namely, differences in the prevalence of retinal signs according gender [16].
Other influencing factors are age and ethnicity. In the Blue Mountains Eye Study (BMES) [14] and the Atherosclerosis Risk in Communities (ARIC) Study [20], the prevalence of retinopathy increased with advancing age, whereas in the CHS only some retinal signs revealed an age-dependent relationship [16]. Regarding ethnicity, an enhanced prevalence of retinopathy was suggested in Afro-Caribbeans compared to Europeans, but in this study the use of standardized protocols was not clearly outlined [12]. In the ARIC study higher prevalence of retinopathy has been documented in blacks compared to whites [17]; however this difference was largely explained by the severity of hypertension.
Much less data are available addressing the frequency of new retinal signs. In the Beaver Dam Eye Study (BDES), the 5-year incidence of focal arteriolar narrowing was 7.7 % and of retinopathy 4.6 %, respectively, in normotensive (BP <160/95 mmHg) participants. Both incidences were about doubled in hypertensive patients [13]. In contrast, in the BMES the 5-year incidence for retinopathy was numerically higher in normotensive (BP <140/90 mmHg) subjects (8.2 %) but not clearly increased in hypertensive subjects (10.4 %) [15]. On the other hand, there is good evidence from several epidemiological studies that retinal alterations (i.e., generalized arteriolar narrowing) precedes the development of hypertension (Table 11.3), as a preclinical marker of hypertension.
Table 11.3
Large-scale, population-based studies (in alphabetical order) assessing associations between retinal vascular caliber (based on retinal photography) and blood pressure, target-organ damage, and cardiovascular risk (in chronological order)
Study | Ref. | Country | Ethnicity | Year | Sample size | Retinal vascular | Finding |
|---|---|---|---|---|---|---|---|
Atherosclerosis Risk in Communities (ARIC) Study | [21] | USA | White, black | 1999 | 9,300 | AVR ↓ | Past and current blood pressure |
[22] | 2001 | 10,358 | AVR ↓ | Incident stroke | |||
[23] | 2002 | 9,648 | AVR ↓ | Incident CHD, acute MI (only in women) | |||
[24] | 2004 | 5,628 | AVR ↓ | Incident hypertension | |||
[25] | (Only) black White, black | 2008 | 1,439 | CRAE ↓ | Left ventricular hypertrophy | ||
AVR ↓ | Left ventricular hypertrophy | ||||||
[26] | 2010 | 10,496 | CRAE ↓ | Incident lacunar stroke | |||
CRVE ↑ | Incident lacunar stroke | ||||||
Beaver Dam Eye Study (BDES) | [27] | USA | White | 2003 | 1,611 | AVR ↓ | CV mortality (43–74 years) |
[28] | 2003 | 4,926 | CRAE ↓ | Current blood pressure | |||
AVR ↓ | Current blood pressure | ||||||
[29] | 2004 | 2,451 | AVR ↓ | Incident hypertension | |||
[30] | 2007 | 4,926 | CRAE ↓ | CHD death | |||
CRVE ↑ | CHD death | ||||||
Blue Mountains Eye Study (BMES) | [31] | Australia | White | 2003 | 3,654 | CRAE ↓ | Current blood pressure |
CRVE ↓ | Current blood pressure | ||||||
AVR ↓ | Current blood pressure | ||||||
[32] | 2004 | 2,335 | CRAE ↓ | Past and current systolic/diastolic blood pressure | |||
AVR ↓ | Past diastolic and current systolic/diastolic blood pressure | ||||||
[33] | 2004 | 1,319 | CRAE ↓ | Incident severe hypertension | |||
AVR ↓ | Incident severe hypertension | ||||||
[34] | 2006 | 3,654 | CRVE ↑ | CHD death (men and women, 49–75 years) | |||
CRAE ↓ | CHD death (women, 49–75 years) | ||||||
AVR ↓ | CHD death (women, 49–75 years) | ||||||
Cardiovascular Health Study (CHS) | [35] | USA | White, black | 2002 | 2,405 | CRAE ↓ | Past and current blood pressure |
AVR ↓ | Current blood pressure | ||||||
[36] | 2006 | 1,992 | CRAE ↓ | Incident CHD | |||
CRVE ↑ | Incident CHD and stroke | ||||||
AVR ↓ | Incident CHD | ||||||
Multi-Ethnic Study of Atherosclerosis, (MESA) | [37] | USA | White, Hispanics, black, Chinese | 2006 | 5,979 | CRAE ↓ | Current blood pressure |
[38] | 2009 | 2,583 | CRAE ↓ | Incident hypertension | |||
CRVE ↑
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|