CT Evaluation of Myocardial Perfusion, Function, and Late Enhancement
Hildo J. Lamb
Lucia J.M. Kroft
Albert de Roos
INTRODUCTION
Cardiovascular CT (CVCT) for evaluation of coronary artery disease has become a routine clinical application. Once a patient has a clinical indication for coronary CT angiography, additional CT acquisitions can be considered. In principle, ECG-gated CT examinations allow acquisition of dynamic information such as myocardial function. However, dynamic CT examinations require a substantially higher radiation dose, mainly because more than one cardiac time frame has to be imaged to be able to evaluate myocardial motion. On the other hand, sometimes ECG-triggered CT acquisitions are performed for other reasons, for example, in patients with high heart rate, where the optimal reconstruction phase is difficult to predict. In those patients functional information is available as part of their standard CT examination and therefore needs to be evaluated.
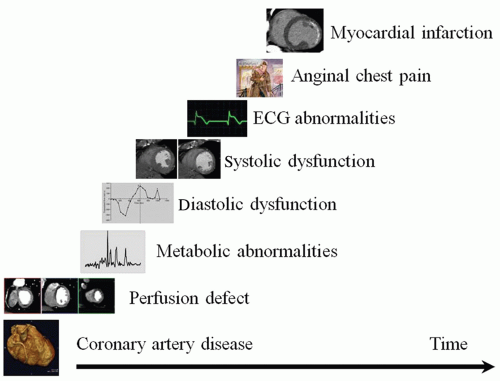
Figure 26.1 shows a schematic diagram of the ischemic cascade. This scheme is used to discuss CVCT applications outside the scope of coronary angiography. Some aspects of the ischemic cascade cannot be evaluated with CVCT, for example, metabolic abnormalities cannot be derived from CT data, or will be discussed elsewhere, such as CT vessel wall imaging.
MYOCARDIAL PERFUSION
After development of a significant coronary artery stenosis, diminished myocardial tissue perfusion is the next step in the ischemic cascade. Noninvasive anatomic evaluation of the coronary arteries by CT angiography has been accepted as imaging tool in patients with low to intermediate pre-test probability for coronary artery disease (1). However, if a coronary artery stenosis is found, the functional relevance of the stenosis is difficult to predict, as the relationship between the severity of stenosis and occurrence of complaints and/or ischemia is unclear. This mainly indicates that patients with anatomically significant coronary artery stenosis may not have ischemia, for example, because of development of sufficient collateral circulation. This is the case not only for coronary artery stenoses found on coronary CT angiography (2,3) but also for invasive coronary angiography (4,5,6 and 7). Therefore, a functional test is needed to assess the hemodynamic effect of the anatomical stenosis found, which is essential in determining appropriate patient management (2,3,4,5,6 and 7).
Ischemic myocardium can be detected by stress myocardial perfusion imaging (MPI) performed by using vasodilators such as adenosine or dipyridamole. Myocardial stress imaging identifies severe stenosis that reduces maximum blood flow but not resting flow. Rest perfusion can be done to determine whether perfusion defects are fixed, or partly or completely reversible.
Several imaging techniques are clinically available for stress MPI. Today, the nuclear medicine technique singlephoton emission computed tomography (SPECT) is most widely applied to this purpose. Stress MPI using SPECT has shown incremental value to coronary CT angiography
for the detection of coronary artery stenoses that are hemodynamically significant (8). Also, magnetic resonance imaging (MRI) has been shown a robust tool for stress MPI and is used in clinical practice. Currently, CT protocols are developed for stress MPI as well.
for the detection of coronary artery stenoses that are hemodynamically significant (8). Also, magnetic resonance imaging (MRI) has been shown a robust tool for stress MPI and is used in clinical practice. Currently, CT protocols are developed for stress MPI as well.
ADVANTAGES OF STRESS CT MPI
Stress CT MPI has many potential advantages as compared with SPECT or MRI techniques. Major advantages are: (1) Possibility for assessing morphology (stenosis) together with its hemodynamic functional effect (perfusion) in a single CT examination (9,10 and 11); (2) CT has short scan times; the full CT MPI protocol including stress and rest imaging can be performed in 20 minutes (by using intravenous aminophylline and beta-blockers for rest scanning) (12). Stress and rest imaging including late enhancement have even been performed within 20 minutes (10); (3) CT has superior spatial resolution (10,11). Nuclear medicine techniques such as SPECT and positron emission tomography cannot depict transmural differences in myocardial perfusion, while ischemic myocardium is first manifested in the subendocardium. With MRI that has improved spatial resolution, transmural differences can be detected. Preliminary studies have shown that CT can also depict transmural perfusion differences (13). CT may therefore improve the detection of small areas of ischemia or infarction (10); (4) CT potentially allows quantification of myocardial blood flow (11); (5) Stress CT MPI can be particularly useful in cases of limited coronary CT angiography quality (11,14,15), such as in patients with severe calcifications, with stents, in image quality-limiting motion artifacts, or in patients with intermediate stenoses that are difficult to grade regarding hemodynamic consequences. Also, stress CT MPI may be combined with late enhancement imaging for assessing viability (9,14); (6) Stress CT MPI may save costs in combination with coronary CT angiography because it can be performed during a single procedure as compared to multiple procedures with different imaging techniques.
PATIENT PREPARATION
Similar to SPECT or MRI MPI, a full stress CT MPI investigation includes imaging at stress and rest. This requires two antecubital intravenous 18G to 20G cannulas, one for administering the stress agent and one for contrast agent. Usually, vasodilator adenosine is administered, with injection starting 3 to 4 minutes before scan start, until scan finish, with a dose of 0.14 mg/kg/min (140 µg/kg/min). Patients are instructed to withdraw from coffee or tea for 24 hours before examination. During the procedure, the patient is closely monitored for heart rate, blood pressure, and ECG for ischemia. Beta-blockers and/or other medication may be used depending on the protocol used.
STATIC VERSUS DYNAMIC STRESS CT MPI
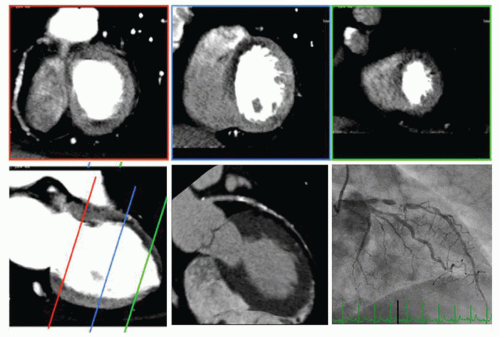
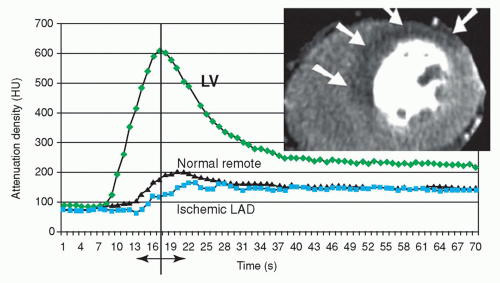
There are two basic techniques that can be applied for stress CT MPI: Static imaging (Fig. 26.2) and dynamic imaging (Fig. 26.3). Roughly, regarding time resolution, static stress CT MPI is analogous to “static” SPECT MPI and dynamic stress CT MPI is analogous to dynamic MRI MPI. Essential is that both static and dynamic CT MPI are performed during the first pass of contrast agent bolus passage through the myocardial tissue. Differences in enhancement between normal and ischemic myocardium are maximum during the upslope of myocardial bolus passage. During the downslope, the differences become less and disappear (Fig. 26.3). This means that after bolus passage, normal and ischemic myocardium cannot be distinguished by enhancement differences anymore (16,17). Exact timing of the scan is therefore of paramount importance.
With static CT MPI or “snapshot imaging,” only a single image stack is acquired such as with coronary CT angiography. Static imaging requires exact scan timing at a single time point during the upslope or maximum of the contrast agent bolus passage. Myocardial blood flow can then be assessed qualitatively and semi-quantitatively. Static CT MPI can be performed by using helical or step-and-shoot techniques.
With dynamic CT MPI or “serial imaging,” multiple image stacks are acquired at multiple time points during the upslope of contrast passage through the myocardial tissue as to obtain a perfusion curve in time (Fig. 26.3). Next to qualitatively and semi-quantitatively flow measures, dynamic imaging potentially allows absolute blood flow calculation (18). Dynamic CT MPI requires advanced CT scanners. At the moment, the technique most often used is shuttle-mode imaging with alternating table positions for covering the left ventricle by moving to-and-fro between consecutive heart beats. This technique results in limited coverage of the left ventricle of 7.3 cm, as well as in contrast passage time delay within image stacks (19,16). Wide-volume scanners have the unique potential to overcome these problems by dynamic scanning with full coverage of the left ventricle without contrast time delay for each time point during first pass perfusion (13).
CT MPI PATTERNS
The CT MPI patterns are basically the same as with other perfusion techniques. In normal myocardium, no perfusion defects will be observed. A perfusion defect due to decreased attenuation observed during stress but not at rest indicates reversible defect (or stress-induced ischemia). A perfusion defect observed at stress and rest indicates fixed defect (or myocardial infarct). In addition, late enhancement may be used for assessment of viability (16,20,21).
CALCULATION OF BLOOD FLOW
With static CT MPI, blood flow or volume calculation is limited to qualitative or semi-quantitative measures. Animal studies have shown strong nonlinear correlation between static CT MPI and microsphere myocardial blood flow (22). Also, by using static stress CT MPI in 40 patients, the transmural perfusion ratio (TPR) has been measured by dividing the subendocardial attenuation density by the subepicardial attenuation density. It was found that TPR was statistical significant inversely and linearly related to percent diameter stenosis, with a correlation of R = -0.63. In addition, abnormal TPR had sensitivity and specificity of 88% and 91% for detecting ischemia as compared to invasive coronary angiography and SPECT MPI (13). It was found that because of the excellent spatial resolution, stress CT MPI allows the detection of subendocardial ischemia.
With dynamic imaging, tissue attenuation curves derived from serial imaging can be used for semi-quantitative or absolute quantification of myocardial blood flow. Valdiviezo et al. (18), have described three upslope evaluation techniques for dynamic imaging. The semi-quantitative method for calculating myocardial blood flow uses linear fitting applied to time-attenuation data by upslope analysis that requires only a portion of the contrast bolus. The myocardial tissue attenuation curve is normalized by the left ventricular blood pool attenuation curve (or, alternatively, by the left ventricle maximum). This method has shown high correlation with microspheres and seems most easy and practical to use.
Absolute measures of myocardial blood flow using modelbased Patlak plot analysis and model-based convolution are more complex and require higher level of expertise. Patlak plot analysis is also used in nuclear medicine MPI and uses an upslope two-compartment model. Modeling with parameters arterial input function (blood pool) in time, myocardial attenuation in time, and the transfer constant of contrast to the extracellular space, is used for calculating myocardial blood flow. For model-based deconvolution, that has initially been developed for MRI, an attenuation-to-iodine concentration curve is produced and used to create timeiodine concentration curves for all tissue attenuation curves as to measure absolute myocardial blood flow. The technique is difficult and requires high level of expertise (18). Still, absolute quantification methods may be limited by assumptions applied with modeling with regard to iodinated contrast and CT.
Absolute measures of myocardial blood flow using modelbased Patlak plot analysis and model-based convolution are more complex and require higher level of expertise. Patlak plot analysis is also used in nuclear medicine MPI and uses an upslope two-compartment model. Modeling with parameters arterial input function (blood pool) in time, myocardial attenuation in time, and the transfer constant of contrast to the extracellular space, is used for calculating myocardial blood flow. For model-based deconvolution, that has initially been developed for MRI, an attenuation-to-iodine concentration curve is produced and used to create timeiodine concentration curves for all tissue attenuation curves as to measure absolute myocardial blood flow. The technique is difficult and requires high level of expertise (18). Still, absolute quantification methods may be limited by assumptions applied with modeling with regard to iodinated contrast and CT.
ACCURACY OF STRESS CT MPI INVESTIGATIONS FOR DETECTING ISCHEMIA
First feasibility studies already have recognized the potential strength of stress CT MPI. Stress-induced myocardial perfusion defects could be identified with diagnostic accuracy and radiation doses comparable to SPECT, but now with the great advantage of visualizing coronary artery stenoses as well (10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree