Critical Pathways Following Thrombolysis
Christopher P. Cannon
Patrick T. O’Gara
The use of fibrinolytic therapy for acute ST-segment elevation myocardial infarction (MI) has dramatically reduced mortality (1). As discussed in chapter 6, an emergency department (ED) pathway for thrombolysis focuses on several components of immediate treatment: (a) rapid time to treatment—both overall time to treatment as well as the door-to-drug time; (b) accurate dosing (i.e., avoiding medication errors); and (c) adjunctive therapy with antiplatelet, antithrombin, and anti-ischemic medications (1). A second component of therapy, however, is the appropriate use of cardiac procedures (i.e., what revascularization strategies are needed following the start of thrombolysis). In addition, hospital length of stay is an area where cost savings can likely be achieved without compromising patient safety.
There are four broad categories of strategies for revascularization: (a) rescue percutaneous coronary intervention (PCI) for patients with evidence of failed thrombolysis (or early reocclusion) where PCI is used to reopen the persistently occluded coronary artery; (b) facilitated PCI, in which a thrombolytic drug (or other pharmacologic agents) is given prior to planned immediate PCI; (c) routine invasive strategy PCI, in which PCI is performed within a few days after thrombolysis; and (d) conservative strategy, where angiography and PCI are performed only if the patient has recurrent ischemia at rest or has evidence of ischemia on stress testing.
Assessment of Early Reperfusion
A key factor in prognosis is the success of early reperfusion. The “open artery theory” notes that if early reperfusion is achieved, the infarct size is reduced, left ventricular (LV) dysfunction is reduced, and survival is improved. One way of quantifying early reperfusion is with angiography, which has been assessed 60 to 90 minutes after thrombolysis in many clinical trials of thrombolysis. Mortality after fibrinolytic therapy is closely related to the degree to which flow has been restored in the infarct-related artery, and the thrombolysis in myocardial infarction (TIMI) flow grading system has been well validated for assessing reperfusion (Table 11-1). In angiographic trials, improved mortality has been seen with patent arteries (TIMI grade 2 or 3 flow), with the lowest mortality among those with TIMI grade 3 flow, which has established this as the ideal goal for early therapy (2).
For clinical care, however, a noninvasive technique is needed; one that may be more useful for evaluating myocardial perfusion is electrocardiogram (ECG) assessment of ST-segment resolution. More than 50% resolution of ST-segment elevation at 60 to 90 minutes has been shown to be a good indicator of enhanced myocardial perfusion and recovery of LV function, reduced infarct size, and improved prognosis (3,4,5). In the TIMI-14 study, ST-segment resolution of more than 70% was a stronger indicator of better survival than TIMI grade 3 flow (5). Other techniques used to assess reperfusion include myocardial contrast echocardiography (6) and myocardial angiographic perfusion with assessment of angiographic blush in the myocardium (7).
The American College of Cardiology/American Heart Association (ACC/AHA) Guidelines suggest that it is reasonable to monitor clinical symptoms, the pattern of ST-segment elevation, and cardiac rhythm over the 60 to 180 minutes after the start of fibrinolytic therapy (1). Noninvasive findings that suggest reperfusion include relief of symptoms, maintenance or restoration of hemodynamic and/or electrical stability, and
a reduction of at least 50% of the initial ST-segment elevation on follow-up ECG done at 60 to 90 minutes. In general, indicators of failed reperfusion include persistent ischemic chest pain, no resolution of ST-segment elevation, and hemodynamic or electrical instability. An invasive strategy (rescue PCI) should be considered for patients with one of these indicators.
a reduction of at least 50% of the initial ST-segment elevation on follow-up ECG done at 60 to 90 minutes. In general, indicators of failed reperfusion include persistent ischemic chest pain, no resolution of ST-segment elevation, and hemodynamic or electrical instability. An invasive strategy (rescue PCI) should be considered for patients with one of these indicators.
Table 11-1. The Timi Flow Grade Classification Used to Assess Early Reperfusion | ||
|---|---|---|
|
Rescue PCI
Rescue PCI refers to PCI that is performed early (usually within 12 hours after fibrinolysis) in patients with evidence of failed reperfusion—thus with a goal of opening the persistently occluded infarct-related artery. There is now a good evidence base of randomized trials to support the addition of rescue PCI into standard clinical care.
The first randomized trial was conducted by Ellis and colleagues, which studied anterior MI patients with a documented occluded artery (8). Those randomized to a rescue PCI strategy done within 8 hours after the onset of symptoms in patients had a lower mortality rate and decreased frequency of a composite endpoint of death or CHF (8). This trial, and observational studies supporting these benefits (9,10), led to recommendations that rescue PCI be considered following thrombolysis, but its uptake in clinical practice has been relatively haphazard.
Two more recent studies have lent much clearer support to the routine incorporation of rescue PCI into critical pathways. One trial randomized 181 patients with STEMI and evidence of failed reperfusion who had been referred for coronary angiography and had TIMI flow grade ≤2 (11). Patients were randomize to undergo coronary stenting or balloon angioplasty, with a primary endpoint of myocardial salvage index, defined as the proportion of initial scintigraphic perfusion defect salvaged by rescue intervention, as assessed 7 to 10 days after PCI. Myocardial salvage index was significantly greater in the stenting group (0.35 versus 0.25; P = 0.005). One-year mortality tended to favor the stenting group, 8% versus 12% (relative risk, 0.6; P = 0.35). This study concluded that patients with failed thrombolysis benefit from rescue PCI in terms of myocardial salvage, with greater myocardial salvage with coronary stenting (11).
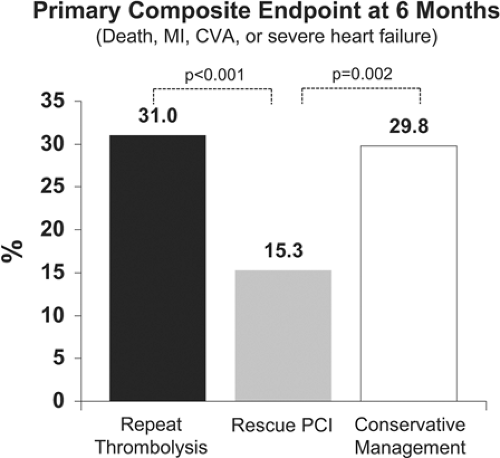
Most importantly, however, the recent REACT trial has reaffirmed the benefit of rescue PCI. This trial studied 427 acute MI patients within 6 hours of pain onset that had failed thrombolysis (diagnosed by <50% resolution of ST elevation on ECG at 90 minutes) (12). They were randomized to conservative treatment, repeat thrombolysis, or rescue PCI. The rate of death, MI, stroke, or severe heart failure at 6 months was significantly reduced with rescue PCI of 15.3% versus 29.8% and 31.0% for the conservative and repeat thrombolysis groups, respectively (each P <0.002) (Fig. 11-1) (12). Thus, rescue PCI should be a part of standard post-thrombolysis care if there are indications of failed thrombolysis. For timing, although benefit of rescue PCI has been seen in these trials when performed on average 6 to 10 hours following thrombolysis, the earlier reperfusion can be achieved the better, and thus efforts are important to shorten time to intervention, as they are for fibrinolysis and primary PCI.
Cardiogenic Shock
One related group of patients falls into this category where early intervention is warranted—patients with evidence of hemodynamic instability. An early invasive approach is recommended for patients in cardiogenic shock, and it may improve outcomes in patients with pulmonary edema (Killip class III). The efficacy of early revascularization in patients in cardiogenic shock following an acute MI was first suggested in an observational study (13) and then demonstrated in the SHOCK trial in patients (14). In the revascularization group, 49% of patients had initially been treated with thrombolytic therapy. The benefit was limited to patients under age 75 in whom 20 lives were saved per 100 treated at 6 months (14). It should be noted, however, that 80% of the patients in this trial had received thrombolysis and/or intra-aortic balloon pumping, and revascularization was carried out 18 to 36 hours following presentation. Thus, this was not a “primary PCI” strategy, but more an early invasive strategy following thrombolysis. According to this strategy, though, critical pathways at hospitals without cardiac catheterization laboratories should include the immediate transfer to a tertiary care center for early revascularization.
Facilitated PCI
Facilitated PCI is a term that encompasses both the pharmacologic therapy and immediate “primary” PCI. It is an attempt to combine the early achievement of an open infarct-related artery with the pharmacologic therapy and the high rates of TIMI grade 3 flow accomplished with PCI. Several types of pharmacologic regimens have been tested: standard (full-dose) thrombolytic therapy, half-dose thrombolytic therapy with a glycoprotein (GP) IIb/IIIa inhibitor, or a GP IIb/IIIa inhibitor alone.
The rationale came from the importance of early TIMI grade 3 flow. In addition, some evidence supporting the notion of facilitated PCI came from an analysis of several of the primary angioplasty in myocardial infarction (PAMI) trials of primary PCI (15). Of these patients, 16% had TIMI grade 3 flow before PCI, and their 6-month mortality was just 0.5% as compared with 2.8% and 4.4% for patients with TIMI grade 2 and 0/1 flow, respectively, at the time of the start of PCI (15). Thus, it could be reasoned that if you could increase the rates of TIMI 3 flow before PCI, mortality would be improved.
Initial trials performed in the 1980s, such as TAMI-I and
TIMI IIA, compared angioplasty performed immediately after thrombolysis to delayed PCI at 18 hours to 10 days as indicated (16,17,18). There was no benefit on LV function, the primary endpoint, and trends toward harm on bleeding, recurrent ischemia, need for coronary artery bypass graft (CABG), and even higher mortality (7% versus 3%) in a European trial (16,17,18). However, since that time there have been many advances in management, including the routine administration of aspirin, GP IIb/IIIa inhibitors and thienopyridines, and stents, and thus many trials were initiated to re-examine this issue. A number of studies, mostly nonrandomized, have suggested that facilitated PCI may be beneficial (19).
TIMI IIA, compared angioplasty performed immediately after thrombolysis to delayed PCI at 18 hours to 10 days as indicated (16,17,18). There was no benefit on LV function, the primary endpoint, and trends toward harm on bleeding, recurrent ischemia, need for coronary artery bypass graft (CABG), and even higher mortality (7% versus 3%) in a European trial (16,17,18). However, since that time there have been many advances in management, including the routine administration of aspirin, GP IIb/IIIa inhibitors and thienopyridines, and stents, and thus many trials were initiated to re-examine this issue. A number of studies, mostly nonrandomized, have suggested that facilitated PCI may be beneficial (19).
Full-Dose Fibrinolytic Therapy Alone
The use of full-dose fibrinolytic therapy as the “facilitating” pharmacologic agent before PCI was tested in several trials, but most definitively in the ASSENT-4 PCI trial (20). A total of 1,667 patients with large STEMIs presenting within 6 hours were randomized to either standard PCI or full-dose tenecteplase (TNK) followed by PCI (that was carried out quickly—on average approximately 1 hour postrandomization). They all received aspirin and unfractionated heparin; GP IIb/IIIa inhibitors were discouraged in the thrombolytic arm, but allowed in the primary PCI arm. The trial was stopped early by the Data and Safety Monitoring Board because of a higher rate of mortality in the pretreatment arm (6% compared with 3%). The primary endpoint—death, congestive heart failure, or shock within 90 days—occurred significantly more frequently in the facilitated PCI arm (18.8% versus 13.7%) (20) (Fig. 11-2). In addition, higher rates of reinfarction (6% versus 4%), repeat target revascularization (7% versus 3%), and stroke (1.8% versus 0%), half of which were intracranial hemorrhages, were seen in the facilitated PCI group. Thus, contrary to the hypothesis, the use of a thrombolytic to open the artery appeared to worsen outcomes, potentially by the known prothrombotic effects of thrombolysis.
Combination of Fibrinolytic Therapy and GP IIb/IIIa Inhibition
A related strategy that might overcome the prothrombotic effects of thrombolysis has also been tested with the use of half-dose fibrinolytic therapy combined with GP IIb/IIIa inhibition before PCI. This unfortunately has not looked promising thus far.
A small randomized trial, the BRAVE study, evaluated whether early administration of the combination of half-dose reteplase plus abciximab produced better results compared with abciximab alone in patients with acute MI referred for PCI (21). The primary outcome measure was assessment of the final infarct size according to a single-photon emission computed tomography perfusion imaging performed between 5 and 10 days after randomization in the 228 patients studied. As expected, patients who received combination therapy had higher coronary patency rates than those treated with abciximab alone (40% vs. 18%, respectively) at the time of initial angiography. This angiographic advantage, however, did not translate into improved myocardial salvage as measured by nuclear scintigraphy. Moreover, bleeding rates were higher in the group receiving the combination of reteplase and abciximab prior to PCI (21). Nearly identical results were seen in another small trial, the ADVANCE MI trial. This trial of just 151 patients also found higher rates of mortality or heart failure with the combination therapy (half-dose fibrinolytic and GP IIb/IIIa inhibitor) than for treatment with a GPIIb/IIIa inhibitor alone (22). The rate of major bleeding was also substantially increased (22). Additional studies are ongoing, but to date this strategy is not encouraging. Thus, based on current evidence, it appears that one needs to choose a single strategy—thrombolysis or primary PCI, but not both.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree