The role of exercise testing to risk stratify patients with repaired coarctation of the aorta (CoA) is controversial. Concentric left ventricular (LV) hypertrophy, defined as an increase in the LV mass-to-volume ratio (MVR), is associated with a greater incidence of adverse cardiovascular events. The objective of the present study was to determine whether a hypertensive response to exercise (HRE) is associated with increased LVMVR in patients with repaired CoA. Adults with repaired CoA who had a symptom-limited exercise test and cardiac magnetic resonance imaging examination within 2 years were identified. A hypertensive response to exercise was defined as a peak systolic blood pressure >220 mm Hg during a symptom-limited exercise test. The LV mass and volume were measured using cardiac magnetic resonance by an investigator who was unaware of patient status. We included 47 patients (median age 27.3 years, interquartile range 19.8 to 37.3), who had undergone CoA repair at a median age of 4.6 years (interquartile range 0.4 to 15.7). Those with (n = 11) and without (n = 36) HRE did not differ in age, age at repair, body surface area, arm-to-leg systolic blood pressure gradient, gender, or peak oxygen uptake with exercise. Those with a HRE had a greater mean systolic blood pressure at rest (146 ± 18 vs 137 ± 18 mm Hg, p = 0.04) and greater median LVMVR (0.85, interquartile range 0.7 to 1, vs 0.66, interquartile range 0.6 to 0.7; p = 0.04) than those without HRE. Adjusting for systolic blood pressure at rest, age, age at repair, and gender, the relation between HRE and LVMVR remained significant (p = 0.001). In conclusion, HRE was associated with increased LVMVR, even after adjusting for multiple covariates.
Despite anatomic repair, patients with coarctation of the aorta (CoA) remain at risk of systemic arterial hypertension, in part, because of abnormalities in vascular structure and function present at birth that persist throughout life. Although many adults with repaired CoA are normotensive at rest, up to 1/3 of tested subjects have an exaggerated hypertensive response to exercise (HRE). Since the 1970s, clinicians have used the blood pressure (BP) response during exercise testing to evaluate asymptomatic normotensive patients with repaired CoA. Despite widespread use and inclusion of exercise testing in recently published guidelines for the care of patients with repaired CoA, scant data are available correlating an HRE to either adverse events or validated intermediate-risk markers for future cardiovascular events. Concentric left ventricular (LV) hypertrophy is a physiologic response to increased afterload and contributes to hypertension through abnormalities in ventricular–arterial coupling. Concentric LV hypertrophy is a well-validated risk marker for increased cardiovascular morbidity, including coronary artery disease and cerebral vascular accidents and is present in 8% to 12.5% of patients with repaired CoA. The present investigation evaluated subjects with repaired CoA to determine whether HRE is associated with concentric LV hypertrophy.
Methods
The committee on clinical investigations at Boston Children’s Hospital approved the study.
All subjects aged ≥16 years with either surgical or transcatheter repair of CoA who had had a symptom-limited exercise test and cardiac magnetic resonance imaging (CMR) examination within 2 years of each other from 1998 to 2008 were identified through a search of the Boston Children’s Hospital cardiovascular database. The exercise tests and CMR were performed as clinically indicated studies as determined by the treating physicians.
Subjects were excluded if they had hemodynamically significant re-coarctation that was clinically determined to require either catheter or surgical reintervention or had collateral vessels identified on CMR. They were also excluded if they had associated complex congenital heart disease, an interrupted aortic arch, aortic valve dysfunction (valve area <1.5 cm 2 , peak velocity by continuous wave Doppler echocardiography of ≥3 m/s, or moderate or greater aortic regurgitation [regurgitation fraction ≥20%]), or other features that would predispose to increased LV mass. Subjects with known genetic syndromes (e.g., William or Turner syndrome) or inherited cardiomyopathy (e.g., Fabry or Gaucher disease) were also excluded.
Demographic data were extracted from the medical records and included age, age at initial repair, number and type of interventions, gender, race, use and type of antihypertensive medications, and bicommissural status of the aortic valve.
Baseline BP was measured with the subject supine, after ≥2 minutes of rest as a part of the standard clinical protocol for cardiopulmonary exercise testing. Arm and leg BP measurements were taken using an automated cuff (Dinamap, Milwaukee, Wisconsin) with the patient supine. Exercise BP was measured in the arm using a manual cuff at 3-minute intervals. The peak exercise systolic BP was defined as the greatest systolic BP measured either during exercise or in the immediate recovery period. Subjects who exhibited a peak systolic BP >220 mm Hg were defined as having a hypertensive response to exercise (HRE positive). Exercise diastolic BP was not reported, because these measurements have been recognized to be less reliable. Patients were instructed to take their prescribed antihypertensive medications on the day of the exercise test.
Exercise testing was performed on either a progressive bicycle ergometer or a treadmill using the standard Bruce protocol. A subset of subjects also completed metabolic cart testing with baseline spirometry followed by breath-by-breath analysis of expiratory gases using a metabolic cart (Medical Graphics, St Paul, Minnesota) and continuous electrocardiographic monitoring. The subjects were excluded if they failed to reach a peak respiratory exchange ratio ≥1.1 (indicating submaximum effort) or if the reason for termination of the exercise test was other than fatigue.
The CMR protocol used in our laboratory for assessment of patients with repaired CoA has been previously published. In brief, CMR was performed on a commercially available 1.5-T scanner (Philips Healthcare, Best, The Netherlands). Electrocardiogram-gated, breath-hold steady-state, free precession images were acquired in short-axis view from the LV apex to its base, with a slice thickness of 8 to 10 mm. LV endocardial and epicardial contours were traced in end-diastole using dedicated software (QMass, version 7.4, Medis, Leiden, The Netherlands). The LV volumes were calculated using Simpson’s rule, and the ejection fraction was calculated as (end-diastolic volume − end-systolic volume)/end-diastolic volume. The LV mass was calculated by multiplying the myocardial area by the slice thickness plus the image gap and multiplying by the specific gravity of the myocardium (1.05 g/ml), as previously described. Concentric LV hypertrophy (i.e., LV mass-to-volume ratio [LVMVR] >0.8 g/ml) has been defined by our CMR laboratory as the cutoff for abnormal findings by consensus and evaluation of historical data from our center. To eliminate the effect of interobserver variability, all contours were traced by a single experienced investigator (A.M.V.) who was unaware of the patients’ clinical status and the results of the exercise test. Phase-contrast flow and velocity imaging was used to quantify significant valve disease, when present.
The primary outcome was the presence of concentric LV hypertrophy. The potential predictors of interest included systolic BP at rest and peak exercise BP. The covariates included age at CMR, body surface area, gender, age at the initial coarctation repair, arm-to-leg BP gradient at rest, and number of surgical or transcatheter CoA interventions.
Normally distributed data are reported as the mean ± SD and non-normally distributed data as the median and interquartile range. Continuous variables were compared between those with and without HRE using Student’s t test or Wilcoxon rank sum test, as appropriate. Fisher’s exact test was used to compare proportions between groups for dichotomous variables. Pearson’s correlation was used to assess the relation between normally distributed continuous variables. We used multivariate linear regression analysis, adjusting for age, gender, body surface area, presence of bicommissural aortic valve, systolic BP at rest, age at first CoA repair, and use of any antihypertensive medication to further investigate the relation between the exercise systolic BP response and LV mass and geometry. Statistical analyses were performed using SAS for Windows, version 9.2 (SAS Institute, Cary, North Carolina).
Results
We initially screened 385 patients with a diagnosis of CoA and CMR examination at our institution during the study period. We excluded 21 patients because of unrepaired CoA (n = 14), significant aortic valve disease (n = 5), interrupted aortic arch (n = 1), or William syndrome (n = 1). We then cross-referenced the electronic medical record to identify those patients who had undergone a symptom-limited exercise test within the study period (n = 59). We excluded patients with an interval of >2 years between the tests (n = 10) and those who were aged <16 years at exercise testing (n = 2), leaving a study cohort of 47 patients meeting the inclusion criteria for additional analysis. The mean interval between the CMR examination and exercise test was 141 ± 185 days; 16 patients underwent both tests on the same day and 6 patients had an interval of 1 to 2 years between the 2 tests.
The baseline demographic and clinical variables and exercise and CMR data are summarized in Table 1 . Initial CoA repair was performed using end-to-end anastomosis in 17, subclavian flap repair in 5, conduit placement in 2, patch aortoplasty in 7, angioplasty without stent placement in 3, angioplasty with stent placement in 7, and an undefined operative strategy from medical record review in 6.
| Variable | Hypertensive Response | p Value | |
|---|---|---|---|
| Yes (n = 11) | No (n = 36) | ||
| Age (yrs) | 22.5 (20.5–37.4) | 28.5 (17.7–37.3) | 0.26 |
| Male subjects | 7 (64%) | 17 (47%) | 0.49 |
| Age at repair (yrs) | 3.0 (0.2–9.4) | 6.8 (0.5–16.9) | 0.33 |
| Weight (kg) | 75 (67.0–92.4) | 71 (64.2–79.4) | 0.38 |
| Body surface area (m 2 ) | 1.9 (1.8–2.2) | 1.8 (1.7–2.0) | 0.19 |
| Interventions (n) | 1 (1–3) | 1 (1–2) | 0.43 |
| Systolic blood pressure at rest (mm Hg) | 146 ± 18 | 134 ± 17 | 0.04 |
| Arm-to-leg systolic blood pressure gradient (mm Hg) | 14 ± 19 | 3.9 ± 18 | 0.20 |
| Antihypertensive medication | 7 (64%) | 20 (56%) | 0.74 |
| Bicommissural aortic valve | 7 (64%) | 23 (63%) | 0.28 |
| Exercise results | |||
| Peak workload (W) | 172 ± 26 | 180 ± 62 | 0.74 |
| Peak oxygen consumption (ml/kg/min) | 26 ± 5 | 32 ± 11 | 0.26 |
| Peak oxygen consumption (% of predicted) | 80 ± 16 | 88 ± 23 | 0.41 |
| Peak oxygen pulse (ml/beat) | 13 ± 4 | 13 ± 4 | 0.89 |
| Peak oxygen pulse (% of predicted) | 83 ± 14 | 96 ± 18 | 0.20 |
| Peak systolic blood pressure (mm Hg) | 235 ± 3 | 179 ± 4 | <0.0001 |
| Peak exercise arm-to-leg systolic blood pressure gradient (mm Hg) | 68 (58–76) | 50 (33–65) | 0.04 |
| Magnetic resonance imaging results | |||
| Indexed left ventricular end-diastolic volume (ml/m 2 ) | 73 (64–92) | 91 (79–110) | 0.03 |
| Left ventricular ejection fraction (%) | 67 ± 6 | 62 ± 9 | 0.10 |
| Left ventricular mass (g) | 128 (78–217) | 113 (87–144) | 0.35 |
| Left ventricular mass-to-volume ratio (g/ml) | 0.85 (0.71–1.0) | 0.66 (0.55–0.77) | 0.007 |
Of the 47 subjects, 11 (23%) had a HRE and 36 (77%) did not. The HRE-positive subjects had a greater systolic BP at rest than did the HRE-negative subjects. A moderate correlation was seen between the systolic BP at rest and the peak exercise systolic BP (r = 0.52, p = 0.0002). The baseline characteristics were otherwise similar between the 2 groups. The median age, age at initial repair, number of interventions, and body surface area were similar between the 2 groups. The BP gradient at rest from the right arm to right leg was not significantly different statistically in the HRE-positive and HRE-negative groups. Of the HRE-positive subjects, 64% were taking antihypertensive medications compared with 56% of the HRE-negative subjects (p = 0.74). The most commonly prescribed antihypertensive agents were angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. However, the sample size was not adequate to explore the relation between the type of antihypertensive medication and the BP response. An equal proportion had a bicommissural aortic valve in the HRE-positive and HRE-negative groups. The type of repair was not associated with LVMVR (p = 0.4) or LV mass (p = 0.2). However, owing to the small numbers for each type of repair, it is possible that an interaction was not detected. The proportion of HRE-positive patients was not different for patients who exercised on a bicycle versus a treadmill (p = 0.3).
Of the 47 subjects, 28 exercised on a bicycle ergometer and 19 exercised on a treadmill ( Table 1 ). A metabolic cart was used to measure oxygen consumption in 35 subjects. The peak workload achieved was similar between the HRE-positive and HRE-negative subjects. The subjects had mildly diminished exercise capacity, which did not differ between the 2 groups. The patients who exercised on a treadmill had a greater maximum relative oxygen consumption than those who exercised on a bicycle (p = 0.03) When accounting for age, gender, and body surface area, the HRE-positive subjects achieved 80 ± 16% of the predicted peak oxygen consumption compared to 88 ± 23% in the HRE-negative group (p = 0.41). Endurance, as measured by peak workload or maximum relative oxygen uptake, was not modified by the age at repair, repair type, or the use of antihypertensive medications.
All subjects had interpretable CMR studies ( Table 1 ). The LV end-diastolic volumes were similar between the 2 groups. The indexed LV end-diastolic volumes were slightly, but significantly lower statistically, in the HRE-positive group.
Global systolic function was within the normal range for all subjects and was similar between the 2 groups.
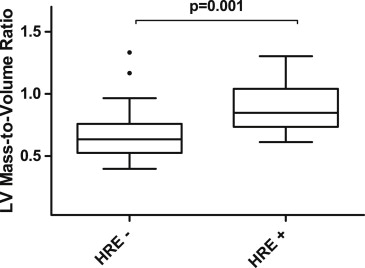
HRE-positive subjects had a greater degree of concentric LV hypertrophy compared to the HRE-negative subjects (median LVMVR 0.85 g/ml, interquartile range 0.71 to 1.0, vs 0.66 g/ml, interquartile range 0.55–0.77, p = 0.007; Figures 1 and 2 ). The odds ratio of having a LVMVR >0.8 was 9.3 (95% confidence interval 2.0 to 43.6) for HRE-positive compared to HRE-negative subjects. When adjusting for systolic BP at rest, age, age at repair, and gender, the relation between HRE and LVMVR remained significant on multivariate analysis (p = 0.001). Additional analysis adjusting specifically for the use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, body mass index, and race did not affect the significance of the primary outcome. No significant interaction was seen between the mode of testing (bicycle ergometer vs treadmill) and the relation between HRE status and LVMVR.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


